What are monoclonal antibodies, and what role could they play in ending a potential pandemic?
This article was updated on 03.07.2024
If you found yourself wandering CEPI's website or perusing our strategy, you would likely soon come across our mission statement: To accelerate the development of vaccines and other biologic countermeasures against epidemic and pandemic threats so they can be accessible to all people in need.
By now, over four years since the World Health Organization declared the COVID-19 pandemic, and over three years since 90-year-old grandmother, Margaret Keenan, became the first person to receive a COVID-19 vaccine outside of clinical trials, the world is acutely aware of the need for lifesaving vaccines and their ability to curb lethal pandemics. But perhaps less obvious is the ‘other' part of our mission statement. That is, the critical role ‘other biologic countermeasures' may play in helping to fight and finish a future disease outbreak.
Regarding epidemic and pandemic response, the term typically refers to a range of countermeasures, including therapeutics, diagnostics and monoclonal antibodies. And it is the latter, monoclonal antibodies — or mAbs — that often has the scientific world abuzz for their potential to tackle a host of diverse diseases.
mAbs have been used safely in medicine for around 30 years, but research into these molecules and their applications has expanded significantly in recent years. From cancer to Ebola to COVID-19 and beyond, mAbs are increasingly helping to rapidly transform how we treat and prevent some of the most stubborn health challenges.
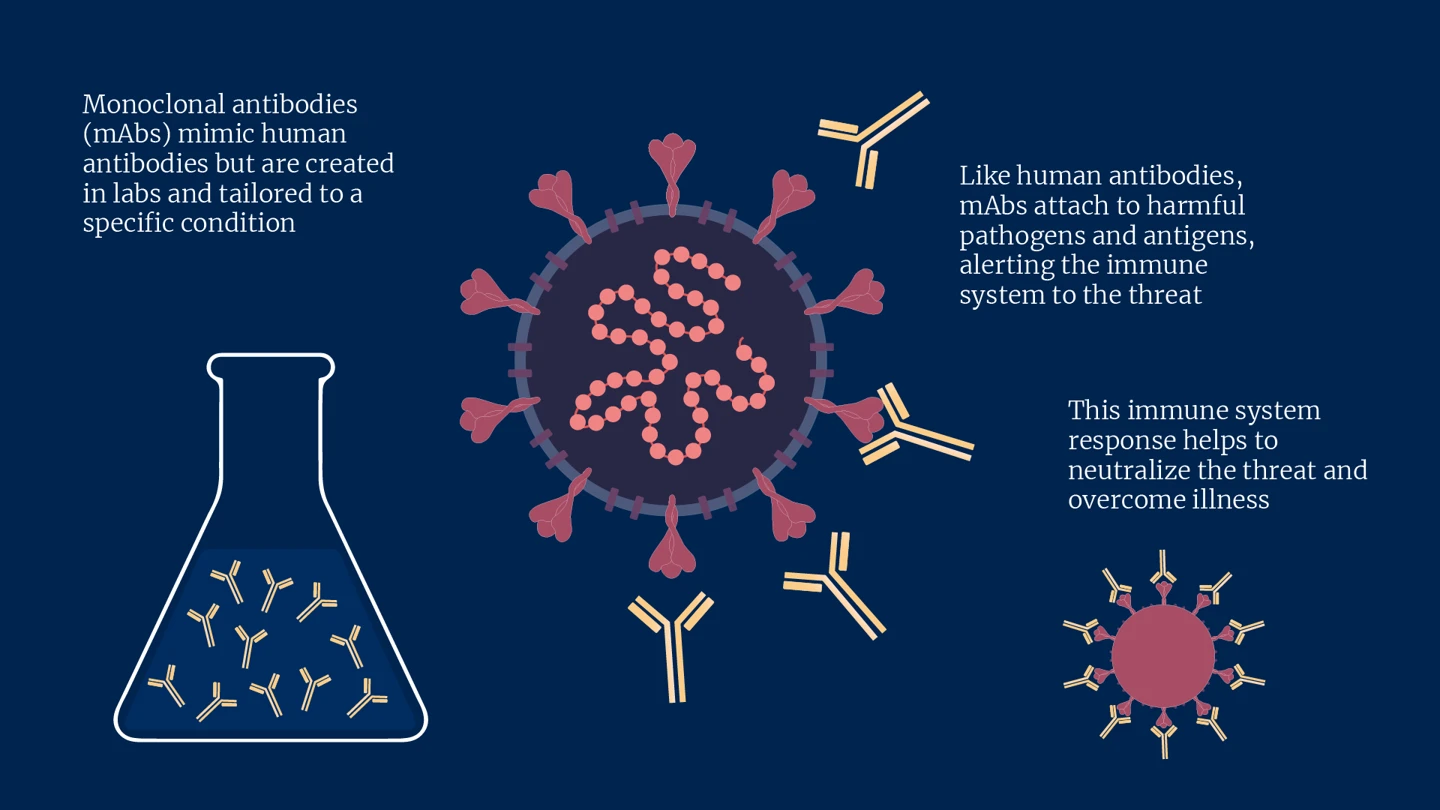
Mimicking the natural antibodies our immune systems produce — which help to fend off disease and infection — mAbs are specifically engineered in laboratories to do the same job but are tailored to a targeted condition. Like human antibodies, they work by attaching themselves to harmful pathogens and antigens and alerting the immune system to the threat, helping neutralise the foreign invader and overcome illness.
CEPI is particularly interested in mAbs for the role they could play in helping to intercept an exponential rise in cases during an emerging disease outbreak. But if a safe and effective vaccine exists against a disease with epidemic or pandemic potential, what role could mAbs play in an outbreak response?
Vaccines are, of course, one of our most powerful weapons against diseases, helping to save over 3 million lives each year globally, and almost wholly eradicating some forms of disease like polio.
That said, it can take weeks for a vaccine-induced immune response to mount to a level sufficient to prevent or reduce severity of infection. During this time, those who have been vaccinated remain unprotected and the dangerous viral pathogen can continue to spread around communities, countries and even continents, exacerbating the potential for an outbreak to balloon into an epidemic or pandemic.
This delay in protective measures could come with catastrophic consequences. The sad reality is that the more people infected, the greater the chance of vaccine scarcity and, therefore, inequity. So, acting with speed and filling any gaps in protection is crucial for a fast and equitable outbreak response.
mAbs, by contrast, can offer immediate protection against infection upon entering the bloodstream, which can last for weeks or even months. The aim of mAbs in an outbreak setting, therefore, is to serve as a bridge, providing instant protection prior to the onset of longer-lasting vaccine-induced immunity. Ideally, such mAbs could be given to people at high risk of exposure, such as healthcare workers and family members or caregivers of an infected person, at the point of vaccination. This would offer much-needed protection during an outbreak of a pernicious disease while a vaccine immune response develops.
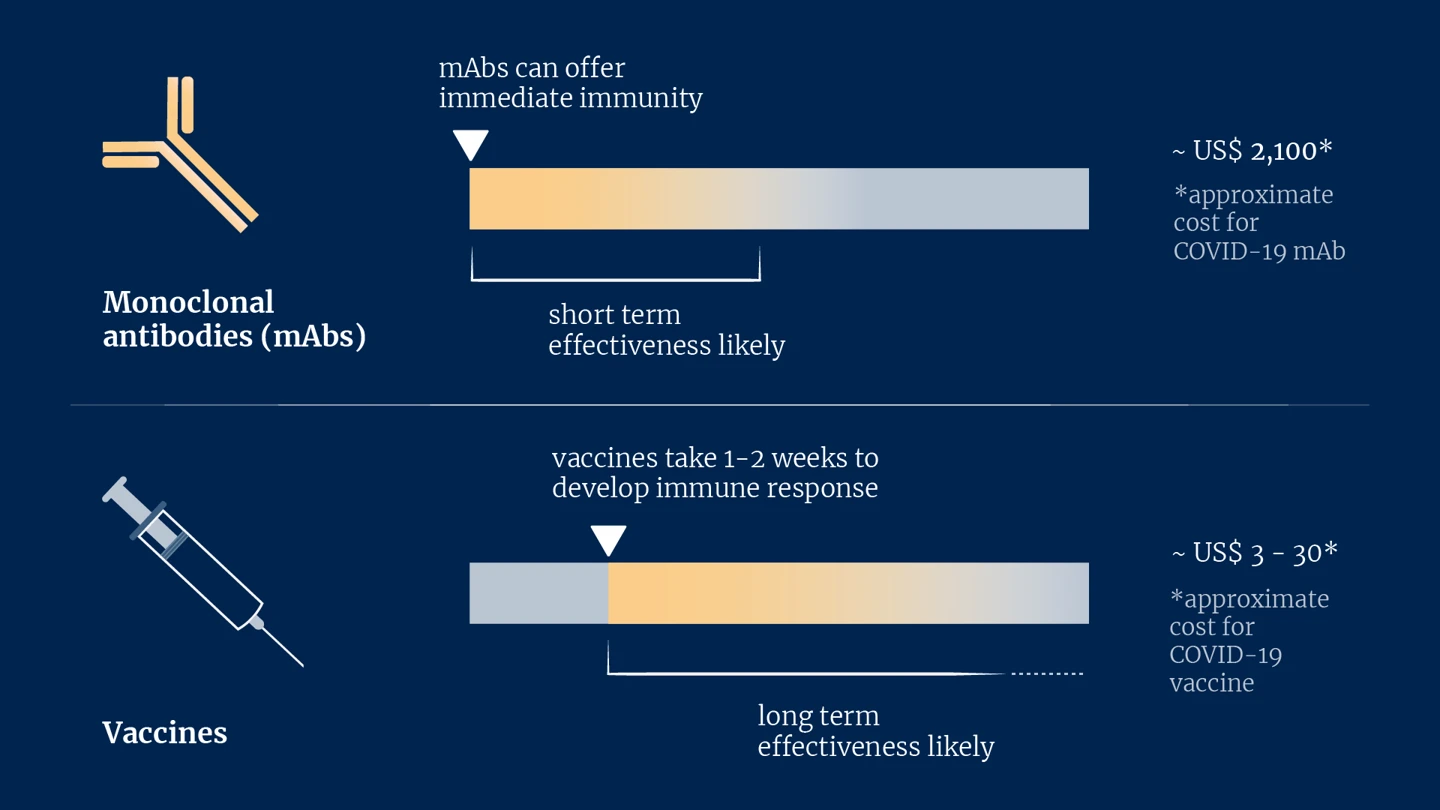
But if mAbs can be so effective, why, then, are they not used more universally? The answer is partly down to money. Currently, mAbs are expensive to produce. Some COVID-19 mAbs, for example, cost around US$2,100 per dose, while most COVID-19 vaccines cost between US$3-30 depending on where you live in the world. Since the immunity mAbs offer tends to wane after weeks and given their present affordability, for now at least, mAbs need to be reserved for the most severe diseases.
That's why CEPI's first major investment in mAbs will be for the deadly Nipah virus. CEPI recently invested in a novel Nipah mAb to advance its development through to human trials in India and Bangladesh in an attempt to provide this immediate layer of protection for one of the deadliest pathogens known to infect humans. Typically spread by fruit bats, Nipah virus kills around 7 in 10 people it infects, and currently has no available medical countermeasures to defend against this disease.
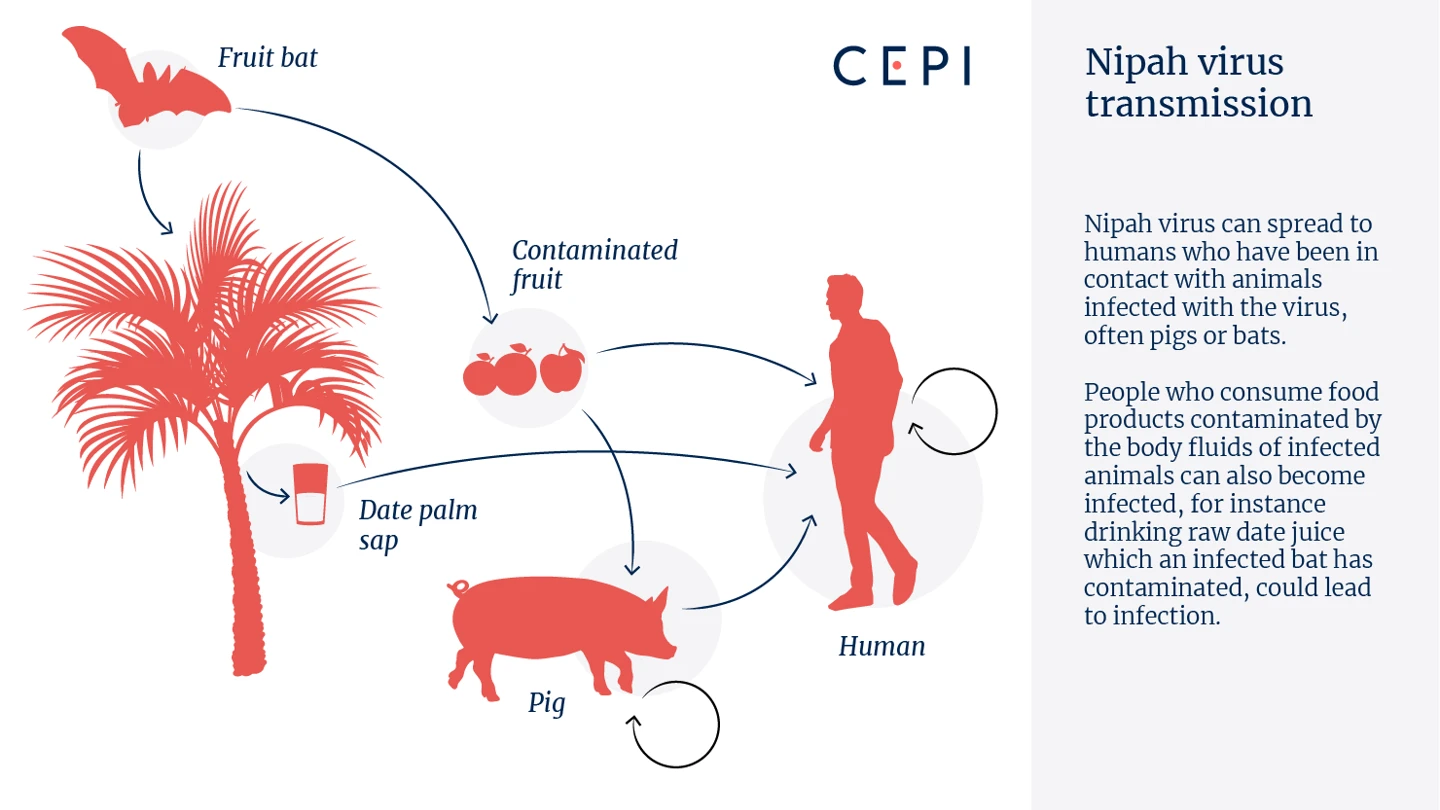
Time left without defences against any disease outbreak carries risks to personal, social and economic well-being, but given how fatal Nipah can be, any time left exposed and unprotected against this virus could be particularly disastrous.
CEPI is the leading global funder of research into developing a vaccine against Nipah virus, one of which was the first-ever to enter clinical trials. Still, even if a Nipah vaccine comes to fruition, the development of a Nipah mAb for pre-exposure prophylaxis (PrEP) — that is, before someone is infected — would be intended for use during an outbreak to contain the virus' spread, while an enduring vaccine response builds. Together these countermeasures, vaccines and mAbs, would offer a potent combination for limiting the devastation that comes with the almost annual Nipah outbreak in endemic countries like Bangladesh and India.
So, in the fight to end pandemics, the entirety of our mission statement soon becomes intelligible. mAbs could play a powerful complementary role, alongside vaccines and other countermeasures, easing the spread of an emerging disease and preventing it from spiralling into the next global pandemic.


.webp)