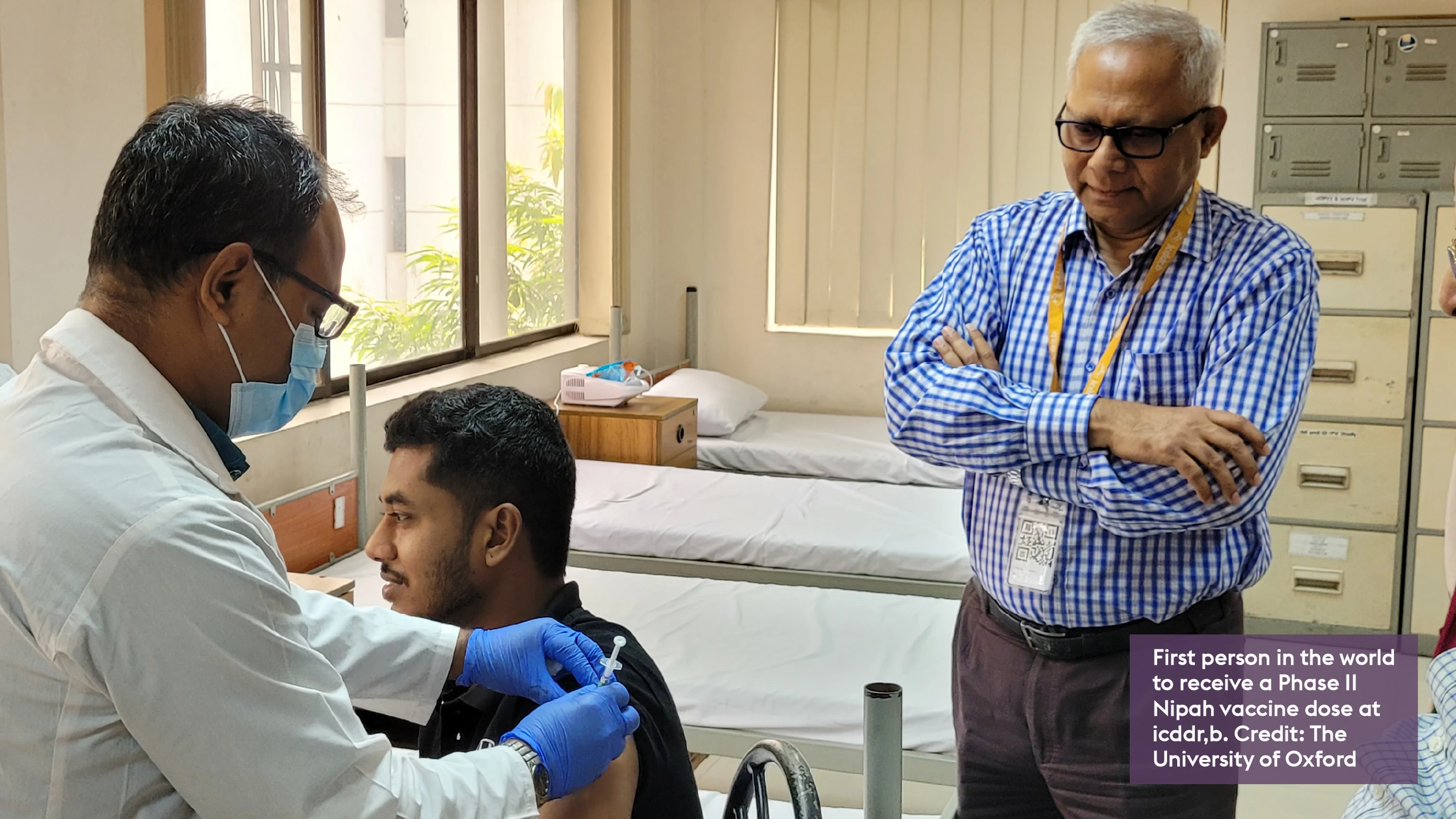
- The University of Oxford has launched the world’s first Phase II clinical trial of a Nipah virus vaccine.
- The trial is being conducted in Bangladesh, a region where Nipah causes regular outbreaks.
- Nipah virus is a deadly zoonotic disease with a case fatality rate of up to 75% and is listed by the World Health Organization as a priority pathogen with pandemic potential for research and development.
9 December 2025. OXFORD, UK. The University of Oxford has launched the world’s first Phase II clinical trial of a Nipah virus vaccine candidate.
The trial, conducted in Bangladesh in partnership with the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), and funded by the Coalition for Epidemic Preparedness Innovations (CEPI), will assess the safety and immune response of the ChAdOx1 NipahB vaccine in a region where the virus causes recurrent outbreaks.
The trial started earlier this month, and will enrol 306 healthy participants aged 18 to 55.
Nipah virus is a deadly disease from the same viral family as measles, the paramyxoviruses, and is recognised by the World Health Organization as a research priority due to its pandemic potential.
A vaccine is urgently needed as the disease can be fatal in up to 75% of cases.
First identified after an outbreak in Malaysia, Nipah virus causes small outbreaks in Bangladesh almost every year, and occasionally in India. Of the 750 cases recorded since 1998, there have been 415 deaths.
The zoonotic virus is carried by fruit bats and its main route of transmission is through drinking contaminated date palm sap. Humans may also be infected via an intermediate animal host, or by person-to-person spread including healthcare workers.
Initial symptoms include fever, headaches, muscle pain, vomiting and sore throat. These can quickly progress to acute encephalitis, pneumonia and severe respiratory problems.
Developed by scientists at the University of Oxford’s Pandemic Sciences Institute, the first-in-human trials of the ChAdOx1 NipahB vaccine started in January 2024 in Oxford, led by the Oxford Vaccine Group. Fifty-one people aged 18 to 55 have safely completed one year of follow-up in the Oxford trial with results expected in the coming months.
The vaccine is made using the same viral vector platform as the Oxford/AstraZeneca COVID-19 vaccine, which is estimated to have saved 6 million lives in its first year alone.
In recognition of the urgent need for a Nipah virus vaccine and the compelling early data, the European Medicines Agency granted the ChAdOx1 NipahB vaccine PRIME (PRIority MEdicines) designation in June 2025. This designation aims to expedite the development and regulatory review processes for medicines that address unmet medical needs.
Commenting on the launch of the trial in Bangladesh, the vaccine’s developer Professor Dame Sarah Gilbert, Professor of Vaccinology at the Pandemic Sciences Institute, University of Oxford said: “This new trial in Bangladesh marks an important step forward in our work to develop a vaccine against Nipah virus, a deadly health threat that currently has no approved vaccine or treatment.”
“The progress we’ve made so far – with the support of our collaborators and funders – is testament to the value of international collaboration and long-term investment in pandemic preparedness.”
Professor Brian Angus, Professor of Medical Practice at the Nuffield Department of Medicine, University of Oxford and Chief Investigator of the trial at the Oxford Vaccine Group, said: “Starting a Phase II trial in a country affected by regular Nipah outbreaks is a critical step in making sure this vaccine is both effective and relevant to the people who need it most. It’s an essential part of ensuring equitable access to protection against emerging infectious diseases.”
Dr Kent Kester, CEPI’s Executive Director of Vaccine Research and Development, said:
“Oxford’s Nipah virus vaccine candidate is the most advanced vaccine against this highly lethal virus. The start of this phase II trial is a first of its kind and represents the culmination of years of cutting-edge research and global scientific collaboration.
“The results from this study will hopefully bring us a step closer towards protecting vulnerable populations against future deadly Nipah outbreaks and will help inform the development of other Paramyxovirus countermeasures.”
Dr K Zaman, Senior Scientist at icddr,b and the Principal Investigator of the trial in Bangladesh, said:“icddr,b has been at the forefront of Nipah virus research for over two decades, operating the world’s longest-running surveillance system and following the largest cohort of Nipah survivors.
“The launch of this world’s first Phase II trial in Bangladesh is therefore not only historic but also a natural progression of our long-standing scientific commitment. By leading this critical study in a country that bears the brunt of Nipah outbreaks, we aim to generate the evidence needed to protect lives from Nipah diseases in Bangladesh and globally.”
The ChAdOx NipahB vaccine was manufactured for this clinical trial by the Serum Institute of India Pvt. Ltd. (SIIPL), part of Cyrus Poonawalla Group, the world’s largest vaccine manufacturer, in collaboration with the Coalition for Epidemic Preparedness Innovations (CEPI).
ENDS
For more information or to request an interview contact:
- Charlie Firth, Communication and Global Engagement Lead, Oxford Vaccine Group: [email protected]
- A K M Tariful Islam Khan, Senior Manager, Communications, icddr,b: [email protected]
- Francis Paynter, Senior Communications and Advocacy Manager, CEPI: [email protected]
Notes to editors
The Oxford Vaccine Group is part of the Department of Paediatrics at the University of Oxford. It led the rapid clinical development of vaccinations against COVID-19 in the pandemic and has made significant contributions to knowledge, supporting national and global policy on immunisation over three decades. OVG was founded in 1994 by Professor E. Richard Moxon. It is one of the world’s leading academic vaccine research teams, and has been led by Professor Sir Andrew Pollard since 2001. The OVG undertakes vaccine research spanning basic science and preclinical studies through to epidemiological studies, human challenge models and phase I-III clinical trials. Current research includes the study of vaccines for outbreak pathogens and pandemics, enteric pathogens, bacterial and viral respiratory infections, and use of human challenge models to accelerate vaccine development.
The Pandemic Sciences Institute is an interdisciplinary research institute at the University of Oxford dedicated to confronting the challenge of epidemic and pandemic infectious diseases. We work with academia, industry and public health organisations across the world to create science-led innovations, accelerate understanding, and develop new diagnostics, treatments, vaccines and disease control tools. PSI is hosted by the University’s Nuffield Department of Medicine. www.psi.ox.ac.uk
The University of Oxford has been ranked number 1 in the Times Higher Education World University Rankings for the ninth year running, and number 3 in the QS World Rankings 2024. At the heart of this success are the twin-pillars of our ground-breaking research and innovation and our distinctive educational offer. Oxford is world-famous for research and teaching excellence and home to some of the most talented people from across the globe. Our work helps the lives of millions, solving real-world problems through a huge network of partnerships and collaborations. The breadth and interdisciplinary nature of our research alongside our personalised approach to teaching sparks imaginative and inventive insights and solutions.
icddr,b is a Bangladesh-based international health research institution that strives to solve key public health problems through high-quality scientific research and innovation. Policy-makers and practitioners utilise our evidence and expertise to improve health outcomes and prevent premature death and disability worldwide. Established more than 60 years ago, we continue to provide life-saving services to the people of Bangladesh, and to nurture the next generation of global health leaders.
CEPI is an innovative partnership between public, private, philanthropic, and civil organisations. Its mission is to accelerate the development of vaccines and other biologic countermeasures against epidemic and pandemic threats so they can be accessible to all people in need. CEPI has supported the development of more than 60 vaccine candidates or platform technologies against multiple known high-risk pathogens or a future Disease X. Central to CEPI’s pandemic-beating plan is the ‘100 Days Mission’ to compress the time taken to develop safe, effective, globally accessible vaccines against new threats to just 100 days. Learn more at CEPI.net.
Serum Institute of India Pvt. Ltd, part of Cyrus Poonawalla Group is a global leader in vaccine manufacturing, dedicated to providing affordable vaccines worldwide. Present across 170+ countries, including the US, UK, and Europe, SII holds the distinction of being the world's largest vaccine manufacturer. SIIPL's multifunctional production and one-of-the-largest facilities in Hadapsar & Manjari, Pune, with an annual capacity of 4 billion doses, has saved over 30 million lives over the years.
Founded in 1966, SIIPL's primary mission is to produce life-saving immunobiological drugs, with a particular emphasis on affordability and accessibility. Guided by a strong commitment to improving global health, the company has played a pivotal role in reducing the prices of essential vaccines, such as Diphtheria, Tetanus, Pertussis, HIB, BCG, r-Hepatitis B, Measles, Mumps, and Rubella. Notably, they are the manufacturers of 'Pneumosiil,' the world's most affordable PCV, 'Cervavac' the first indigenous qHPV vaccine in India, and R21/Matrix-M™, the second Malaria vaccine to be authorized for use in children in malaria-endemic regions, ‘MenFive’, the first in the world Pentavalent (ACYWX) Meningococcal Polysaccharide Conjugate Vaccine, approved and WHO-prequalified for use in the pediatric population. Moreover, SIIPL has been at the forefront of the global fight against COVID-19, delivering over 2 billion doses of the COVID-19 vaccine worldwide.
To further expand its global presence and ensure widespread vaccine availability, SII has established Serum Life Sciences Ltd, a subsidiary in the UK and Serum Inc., a subsidiary in the US. Through relentless pursuit of innovation, SII continues to champion the cause of affordable vaccines, making a positive impact on the lives of millions worldwide www.seruminstitute.com


.webp)
