Transforming vaccine manufacturing
By bolstering global manufacturing capacity in underserved regions and harnessing innovative technologies to improve the speed, scale and access of vaccine manufacturing, the world can improve its response to epidemic and pandemic threats.


Advancing manufacturing innovations
Achieving the 100 Days Mission will require transformative innovations in vaccine platform and manufacturing technologies. As part of CEPI’s Innovations to Prepare for Future Epidemics and Pandemics Call for Proposals, CEPI aims to support projects that will advance manufacturing innovations that improve vaccine scalability and equitable access.
Building a global manufacturing network
The unequal global distribution of vaccine manufacturing capacity was a central driving force behind the tragic vaccine inequity that characterised the response to COVID-19. Expanding and diversifying the global footprint of vaccine manufacturing—particularly in underserved regions—is a cornerstone of CEPI’s goal of enabling equitable access to life-saving vaccines, and will be critical to the success of the 100 Days Mission.
CEPI is supporting capability strengthening activities of selected vaccine manufacturers in Global South countries, near areas at high risk of disease outbreaks, with a particular focus on regions where CEPI’s priority pathogens are endemic. In the event of an outbreak, CEPI-backed developers will quickly be able to transfer their technology to pre-selected manufacturers with the right expertise, technology, and optimal geographical position to enable rapid production and equitable distribution of vaccines to affected populations.

Explore CEPI's manufacturing partnerships
Explore CEPI's manufacturing partnerships

Senegal helps to power Africa’s drive for vaccine independence
Find out how Institut Pasteur de Dakar and CEPI are partnering to help scale-up Africa’s capability to produce vaccines, both for routine immunisation and in preparation against a future pandemic pathogen.

Mobilising Brazil’s manufacturing might to support vaccine production in the Global South

Serum Institute of India joins CEPI global network to boost production of affordable outbreak vaccines

CEPI and Bio Farma partnership boosts rapid response vaccine manufacturing for the Global South

CEPI and Institut Pasteur de Dakar announce 10-year partnership to boost manufacturing of affordable vaccines for the Global South

Aspen, CEPI and the Bill & Melinda Gates Foundation expand commitments to improve access to vaccines in Africa
Explore CEPI's manufacturing partnerships
The need for speed in vaccine manufacturing
Innovating and optimising manufacturing processes for rapid initial production and subsequent scaling up will be critical to responding to future pandemic threats and it is a critical enabler of the 100 Days Mission.
CEPI is investing in novel manufacturing technologies and innovations with the aim to significantly reduce the time needed to manufacture a novel vaccine in response to an outbreak. This will enable faster availability of vaccines for clinical trials and initial emergency use in future disease outbreaks, whether epidemic or pandemic.
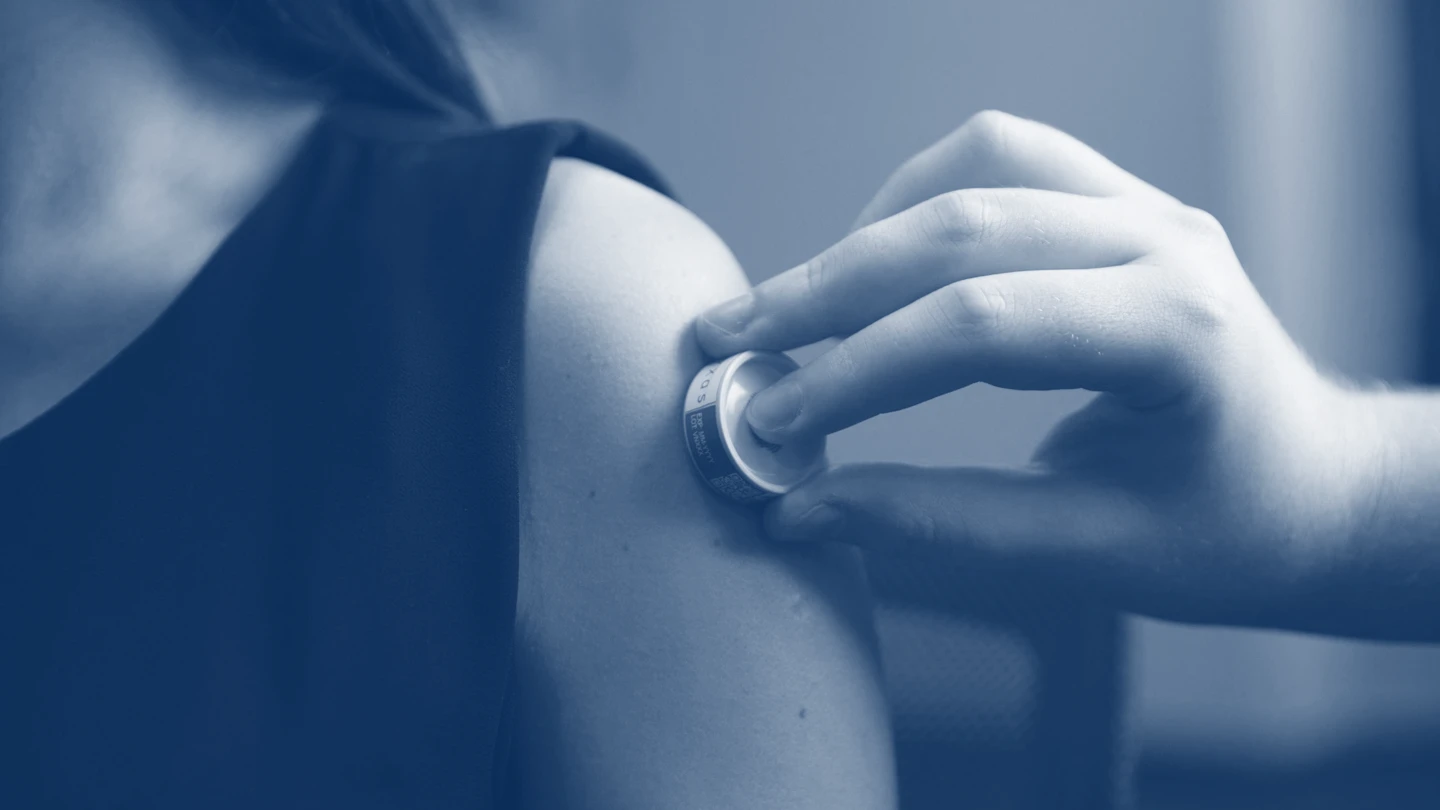
Heat-stabilising vaccines to improve equitable access
Advances in vaccine technology, particularly mRNA technologies, were crucial to ending the acute phase of the COVID-19 pandemic. A major challenge, however, in getting mRNA vaccines to vulnerable populations—especially those in low-resource settings—is the need to store them at very low temperatures, due to the fragility of mRNA molecules.
CEPI is supporting various new heat-stable vaccine platforms, which would minimise the need for complex and costly cold-chain requirements. From needle-free vaccine patches and pens to nanoparticle technology designed to protect fragile mRNA molecules, these innovative vaccine delivery platforms could help to end the need for frozen storage and improve access to potentially life-saving vaccines in the future.

Robust and resilient supply chains
A typical vaccine manufacturing plant will use 9,000 different materials, from 300 suppliers, across 30 countries. From raw materials, like lipids, to vaccine delivery materials, like vials and syringes, a lot goes into making one vaccine. Underscoring globally accessible vaccine manufacturing is, therefore, a robust and reliable supply chain. But with so many moving parts and supply and demand fluctuations, supply-chain constraints and bottlenecks can cause significant delays in access to vaccines.
To overcome these challenges, CEPI supports efforts to streamline and improve the availability of essential materials, equipment and logistics required for vaccine development and manufacturing, connecting suppliers across the world. Enhancing the efficiency and resilience of these supply chains will help the world respond more effectively to emerging infectious diseases.
Chemistry, Manufacturing, and Controls
Chemistry, manufacturing, and controls (CMC) is an essential part of all vaccine development. Manufacturing processes need to be developed to produce vaccines or vaccine candidates, analytical methods are required for testing and release, and the formulations need to be suitable for distribution and delivery. CMC experts support all of CEPI’s vaccine development projects helping to advance platforms for rapid response to outbreaks of emerging infectious threats.
CEPI CMC Rapid Response Framework shows how to accelerate Chemistry, Manufacturing & Controls (CMC) for pandemic-ready vaccines.

Latest manufacturing news
Latest manufacturing news

Senegal helps to power Africa’s drive for vaccine independence
Find out how Institut Pasteur de Dakar and CEPI are partnering to help scale-up Africa’s capability to produce vaccines, both for routine immunisation and in preparation against a future pandemic pathogen.

From coffee pods to moths – the innovation inspirations to help beat pandemics
Explore the vaccine manufacturing innovations helping to realise the 100 Days Mission

Pushing mRNA vaccine development timelines to new speeds

CEPI and Micron Biomedical accelerate needle-free vaccines against Disease X

Transatlantic scientists to transition traditional vaccine development onto rapid-response platform for faster outbreak control

Canadian scientists to optimise vaccine process for faster outbreak response

CEPI partners with Afrigen to speed up mRNA vaccine development and access

Mobilising Brazil’s manufacturing might to support vaccine production in the Global South

Project to explore speed up of mRNA vaccine production deployable for local outbreaks

BioNTech and CEPI expand partnership to strengthen Africa’s mRNA vaccine ecosystem

New ‘spin freezing’ technique could enhance future mRNA vaccines

