Lassa fever
CEPI is the world’s leading funder of research into Lassa fever. As well as bringing the prospect of protection against Lassa fever closer than ever before, CEPI-backed studies are deepening understanding of the Arenavirus family that could one day help prevent the next epidemic.
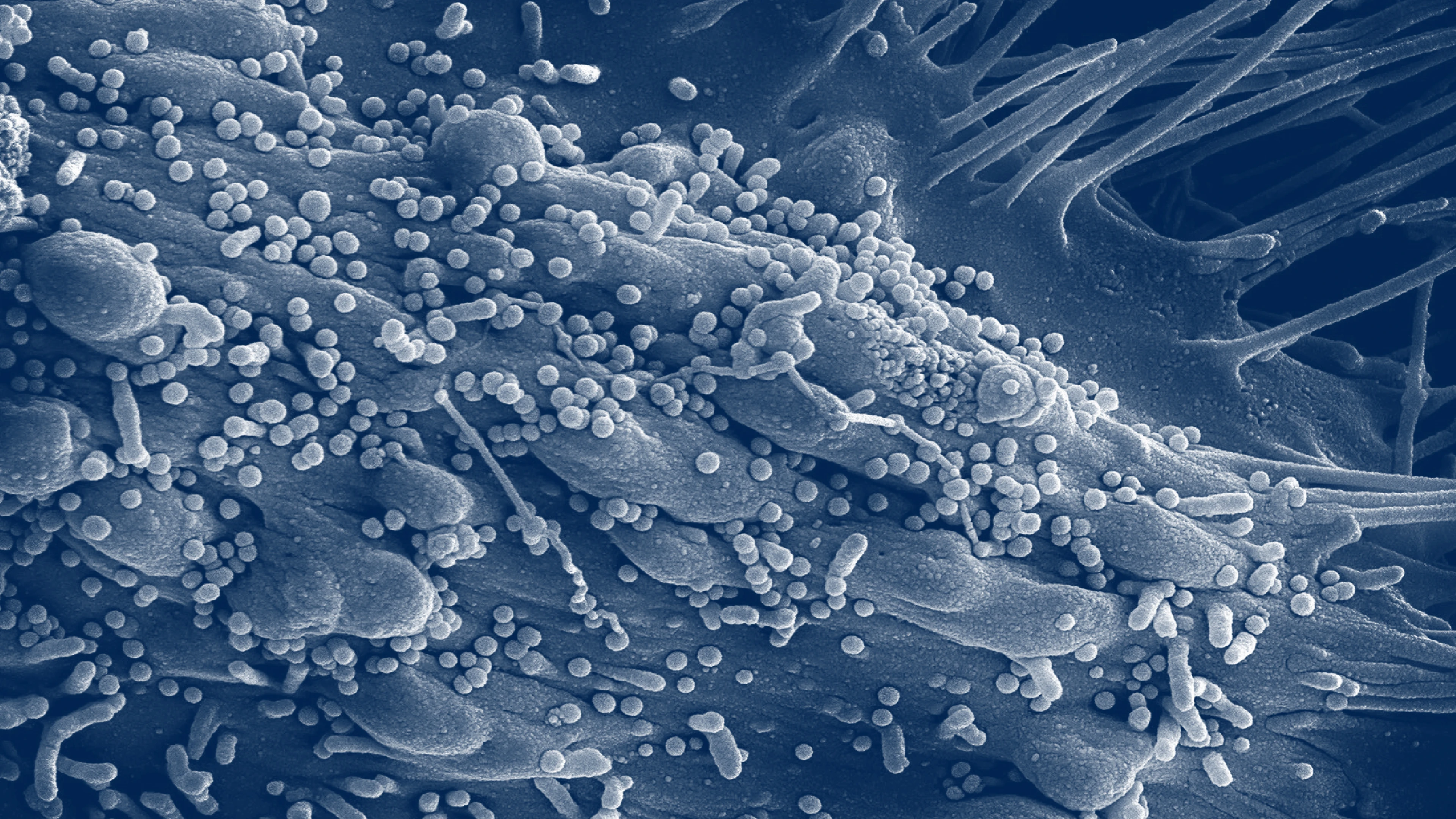
What is Lassa fever?
Lassa fever is a severe viral illness caused by the Lassa virus, a member of the Arenavirus family.
The disease spreads mainly through contact with food or household items contaminated by the urine or droppings of infected Mastomys rats. It can also spread from person to person through direct contact with the blood, tissue, or bodily fluids of someone infected. In some parts of West Africa, Mastomys rats are eaten as food, which increases the risk of infection.
The World Health Organization (WHO) classifies Lassa fever as a priority pathogen because of its potential to cause epidemics and its significant public health impact.
Currently, no vaccines are licensed for Lassa fever. However, CEPI-funded modelling suggests that introducing a safe and effective vaccine across 15 West African countries could save about 3,300 lives over 10 years and prevent up to $128 million in societal costs. More data on the vaccine’s potential impact will be gathered once real-world evidence becomes available.

90-95%
Number of human infections due to indirect exposure or direct contact with infected Mastomys rats
2
CEPI-funded Lassa vaccines in active development
3300
Potential lives saved over 10 years with the introduction of a Lassa vaccine
Where does Lassa fever occur?
Lassa fever occurs regularly in parts of West Africa, including in Benin, Ghana, Guinea, Liberia, Sierra Leone, Togo and Nigeria.
Around 100,000 to 300,000 are thought to be infected with Lassa fever in the region each year, but this is likely a vast underreporting. This is because of difficulties in detecting the virus as symptoms are similar to other diseases affecting the region, like malaria, leading to infections being misdiagnosed. There is also limited access to specialised laboratories in affected countries.
Climate change and growing populations could further amplify extend Lassa's reach and potential impact.

What are the symptoms of Lassa fever?
Most people infected with Lassa virus have no symptoms.
Those who are symptomatic can suffer from mild headache and fever as well as more serious symptoms like vomiting, swelling of the face, pain in the chest and back, and severe bleeding.
Lassa fever can cause severe complications for mother and baby if pregnant women become infected. Around a quarter of survivors suffer from hearing loss.
On average, 1% of cases are fatal.
How is CEPI responding to Lassa fever?
Moving Lassa vaccines towards licensure
CEPI is one of the world’s leading funders of Lassa fever research. We are working with partners in West Africa and across the world to advance one or more Lassa fever vaccines to licensure.
Research funded by CEPI and led by the Universities of Oxford and Liverpool and the Liverpool School of Tropical Medicine found that being vaccinated against Lassa fever would prevent millions of people from falling sick with the disease and facing prohibitive treatment costs that could otherwise push them below the poverty line.
CEPI has invested in five vaccine candidates. Two remain in active clinical development. This includes CEPI's partner IAVI who have launched the first-ever Phase II clinical trial of a Lassa fever vaccine. The CEPI-funded Lassa fever vaccine candidate developed by the University of Oxford recently entered the clinic.
CEPI is also testing rapid-response mRNA vaccine candidates developed by SK Bioscience for use against Disease X. These first mRNA vaccine candidates are using Lassa fever as a representative target for Disease X.
To support the development of a Lassa fever vaccine, CEPI is supporting research seeking to identify the immune markers associated with protection from Lassa fever.

The largest ever study of Lassa fever
CEPI created and funds Enable, the world’s largest ever study of Lassa fever set up to give researchers a better picture of the true disease burden.
Findings from Enable will help us better understand the number of people who are at risk of Lassa virus infection.
Over 23,000 participants have taken part across West Africa since 2019. Enable was expanded in October 2024, with a new study that is taking an in-depth look at Lassa fever symptoms and potential co-infections like malaria.
Insights gained will improve our understanding of Lassa fever and the diversity of symptoms.
This information will guide where and how future late-stage vaccine trials are conducted and help to determine the priority groups who should receive the vaccine once it becomes licensed in the coming years.

Boosting regional collaboration
Regional leadership is critically important to advance the development of vaccines that protect against Lassa fever.
CEPI is supporting the Lassa fever Coalition, a pioneering group established by the West Africa Health Organization (WAHO) to build upon the efforts of national Lassa fever initiatives and ensure regionally those affected by Lassa fever have a vaccine available to protect their populations in the future.
The Lassa Governing Entity—which includes West African Ministers of Health and senior global health representatives—provides country and regional leadership to the Coalition and ensure effective governance and delivery of the programme.
In an historic moment for regional solidarity and leadership, West African Ministers of Health endorsed a communiqué pledging their joint commitment to advance the development of, and readiness for, much-needed vaccines against Lassa fever in September 2025.
CEPI maintains close engagement with institutions including the Economic Community of West African States (ECOWAS) Regional Centre for Surveillance and Disease Control, as well as national public health agencies, such as the Nigeria Centre for Disease Control (NCDC) and Ministries of Health in the most affected countries.
CEPI is also coordinating the ECOWAS RegECs Project, a collaboration between regulators, ethics committees and the African Vaccine Regulatory Forum (AVAREF) to support efficient management of clinical research in West Africa.

On-the-spot Lassa virus diagnostics
CEPI is funding a project led by FIND to examine and evaluate all available point-of-care testing options for Lassa fever virus.
High quality diagnostic tests are essential both for early pathogen detection to stop an outbreak and as part of the vaccine development process - particularly in helping to design the vaccine and conduct clinical trials.
FIND researchers will identify the criteria for an optimum rapid Lassa diagnostic test. Successful diagnostics will be progressed to licensure for widespread use.

Strengthening clinical trial capacity
CEPI is strengthening the infrastructure, equipment and skills needed to run effective late-stage clinical trials of Lassa fever in West Africa.
The work is facilitated by IVI and Medical Research Council Unit The Gambia.
Strengthened clinical trial capacity could allow for the rapid large-scale testing of vaccines locally if the region were faced with a future Disease X.

Preparing for vaccine access
CEPI and the West African Health Organization (WAHO) have launched the first end-to-end access roadmap (available in both English and French) for Lassa fever vaccines. This roadmap sets out a coordinated pathway to ensure that future licensed Lassa vaccines reach the populations who need them most.
The comprehensive resource outlines what needs to take place across the vaccine chain—from early research and development through to policy, manufacturing, procurement, delivery and sustained use— to help health, science and government partners plan for the introduction and rollout of potential future doses years in advance of a licensed product. It will also support Lassa fever vaccine funders and policy-makers in addressing the key needs around vaccine market access.
The resource was created with expert insight from public health officials and Ministers of Health across West Africa, the region where Lassa fever is endemic.
Lassa fever news
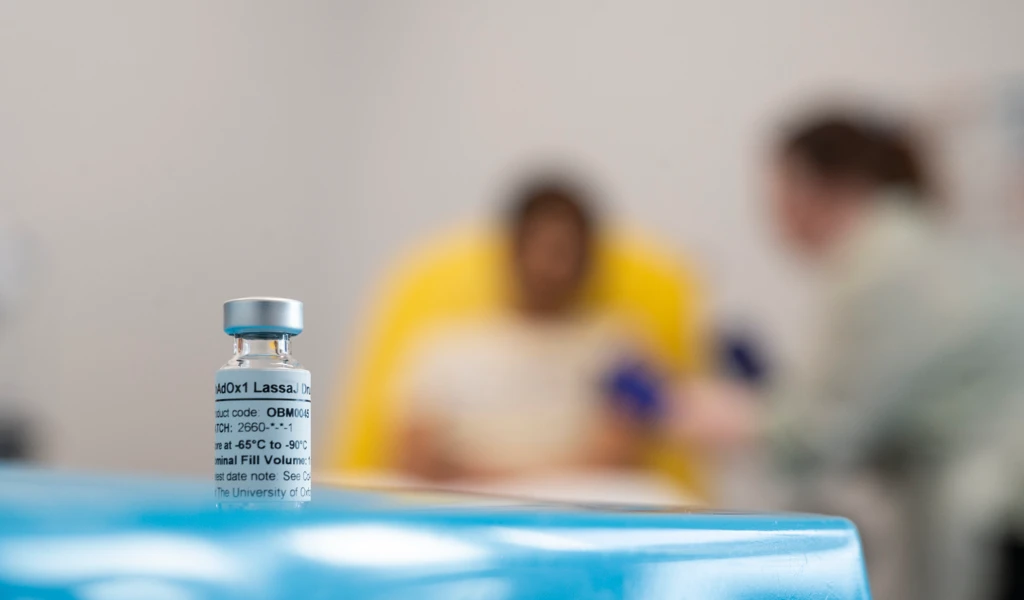
First volunteer receives Lassa fever vaccine in cutting-edge Oxford trial

West African leaders commit to advance Lassa Fever vaccine for the region

Leading health partners join coalition set up to combat deadly Lassa fever
How new vaccine trials in West Africa boost global viral defences

Bringing together all pieces of the Lassa fever vaccine puzzle

Largest-ever Lassa fever study launches in-depth look at disease symptoms

A Lassa vaccine is urgently needed to save thousands of lives
Participants in Nigeria vaccinated in first-ever Phase 2 Lassa fever vaccine clinical trial

Prepare to be prepared: leveraging West Africa's research ecosystem to strengthen outbreak response

As CEPI ramps up vaccine research, a new study warns Lassa fever is set to expand its reach across Africa

