Lassa fever is a human disease caused by a virus that resides within its natural host, the multimammate rat, Mastomys natalensis. Due to the shared living habitat and close proximity of the rodent to people, the virus can infect humans through the exposure with food or household items that have been contaminated by the infected rodent's urine or feces. In addition, Lassa can spread between humans through bodily fluids.
The acute viral disease, which causes hemorrhagic fever, has been on the list of emerging infectious diseases, but has received the spotlight recently after an outbreak in Nigeria in 2018 with over 413 confirmed positive cases and fatality rates exceeding 20%. Although these numbers might appear low and the disease is endemic in countries in West Africa, Lassa fever can easily spread and result in a global outbreak due to exposed travelers or immigrants.
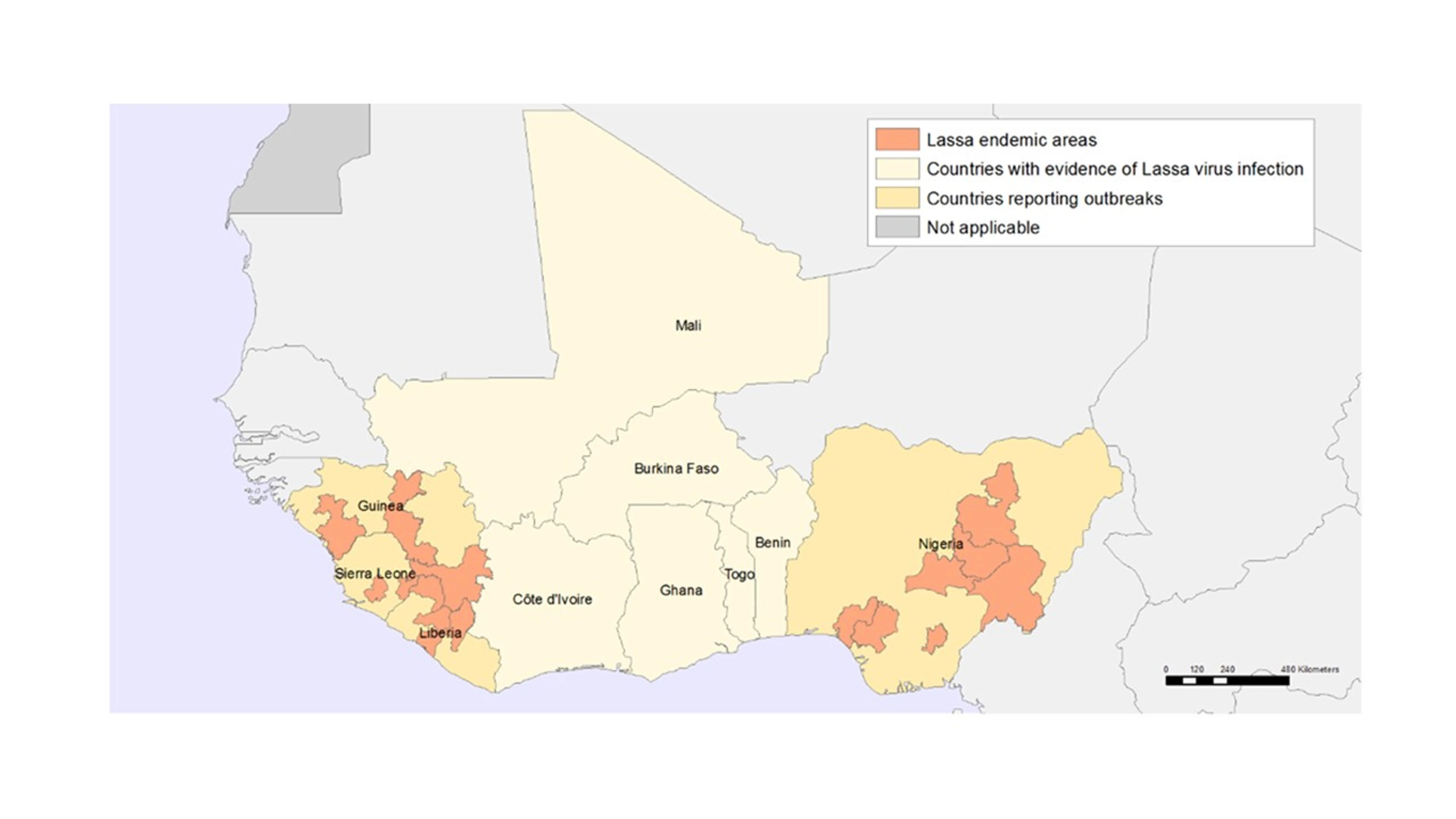
Source: WHO; geographic distribution of Lassa Fever in West African affected countries, 1969 to 2018
Currently, the disease affects up to 300,000 people each year with mortality rates of up to 5,000 deaths annually and about 10%-16% of hospital admissions each year. It is hard to diagnose who has Lassa fever because early symptoms of the disease resemble a flu-like illness that is characterized by fever, sore throat, severe headache and commonly vomiting and nausea. Also, the long incubation time of one to three weeks and the initial mildness of the disease mean that an infected person can get on a plane to a different country without knowing that they could potentially transmit the virus. There are currently no approved vaccines against Lassa fever for use in humans.
What is Themis uniquely bringing to the table to tackle Lassa fever?
Themis just announced the initiation of a Phase 1 clinical trial in healthy adult volunteers to investigate the safety, tolerability and immunogenicity of the MV-LASV vaccine candidate. MV-LASV was developed using a proprietary measles vector platform for prophylaxis of Lassa virus infection. What makes Themis' technology unique is that the delivery vehicle that is used is the measles vaccine vector, which historically has a proven track-record of safety and potency as evidenced by the billions of people who have already been vaccinated world-wide. Themis applies this knowledge to develop innovative vaccine candidates including the one against Lassa fever.
Themis researcher working in the lab
A part of the Lassa virus, which is identified to activate an immune response, is added to the delivery vehicle which then transports it directly to macrophages and dendritic cells, two components of the body's first line of defense against a foreign object, in this case the part of the Lassa virus. This triggers a specific immune response to the Lassa virus and due to the nature of Themis' delivery vehicle, the vaccine is expected to confer long-term immunity.
Why has the CEPI collaboration been so useful?
CEPI is devoted to preparing the world against the emergence of potentially deadly infectious diseases with tremendous outbreak potential. The funding we received has helped us to initiate our Lassa fever program. Organizations like CEPI have stepped in to fund the development of vaccines for diseases that are often overlooked by the pharmaceutical industry due to the large investments needed to develop potential candidates and the potential lower market impact. CEPI's funding will support the development of Themis' Lassa fever vaccine product from pre-clinical stages of testing to clinical evaluation in humans and help advance the vaccine candidate to the clinical proof-of-concept stage where it can be made available immediately in case of an outbreak and provide an immediate impact in preventing global epidemics.


.webp)