Priority diseases
Vaccines can help prevent outbreaks from becoming humanitarian crises. CEPI supports the development of vaccines, platform technologies and manufacturing innovations to combat priority diseases for which no licensed vaccines are currently available.
Targeting diseases with epidemic and pandemic potential
CEPI’s priority pathogens include Chikungunya, COVID-19, Ebola, Lassa Fever, MERS, Nipah, Rift Valley Fever, and novel viral threats with epidemic or pandemic potential (also known as “Disease X”).
In its short history, CEPI has had an outsized influence in shaping the global R&D ecosystem, forging consortia, developing new collaborations and injecting funding to jumpstart R&D when needed. It has also aligned programmes to complement the R&D efforts of its coalition partners.
To date, CEPI has made investments in over 50 vaccine candidates or platform technologies.
Chikungunya
Chikungunya is a mosquito-borne disease, caused by a virus of the same name belonging to the Togavirus family. It is spread to humans through the bites of infected mosquitoes. WHO has highlighted Chikungunya as a major public health risk due to its high morbidity and has stated that further research and development is needed to mitigate the risk it poses.
COVID-19
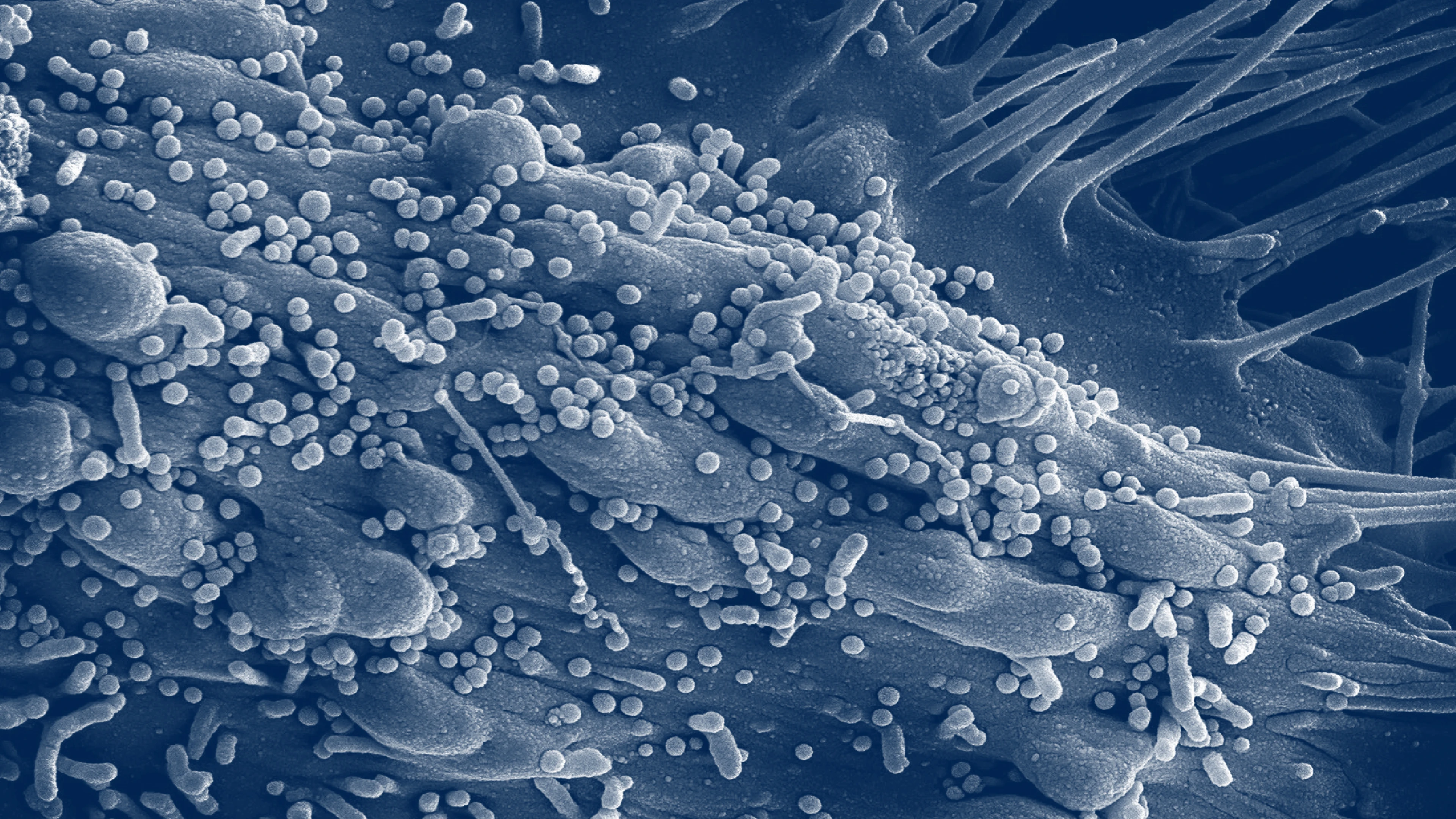
COVID-19 is caused by SARS-CoV-2, a coronavirus related to MERS-CoV. The 21st century has seen three coronavirus epidemics or pandemics already. That is why CEPI is a leading funder of research into broadly protective coronavirus vaccines, which could protect against future SARS-CoV-2 variants as well as other coronaviruses with epidemic and pandemic potential.

Disease X
“Disease X” represents the knowledge that a serious international epidemic could be caused by a pathogen currently unknown to cause human disease. To help the world quickly respond to Disease X, CEPI is funding the development of vaccine platform technologies so that we can rapidly manufacture vaccines against many different types of disease.
Ebola
Ebola virus disease, formerly known as Ebola haemorrhagic fever, is a severe, often fatal illness which affects humans and other primates. It is caused by an infection with a group of viruses within the genus Ebolavirus.
Lassa Fever
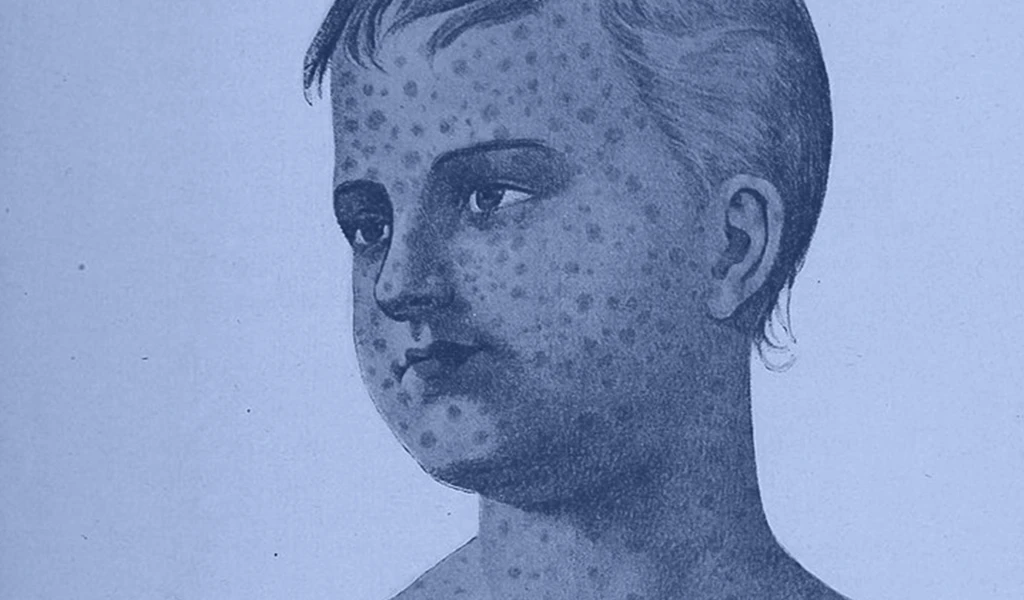
Lassa Fever is an acute viral haemorrhagic disease caused by Lassa virus, belonging to the Arenavirus family. Lassa Fever occurs regularly in parts of West Africa, and is known to be endemic in Benin, Ghana, Guinea, Liberia, Mali, Nigeria, Sierra Leone, and Togo.

MERS
Middle East Respiratory Syndrome (MERS) is a respiratory illness caused by Middle East Respiratory Syndrome Coronavirus (MERS-CoV). This virus belongs to the same family of viruses that causes the common cold, severe acute respiratory syndrome (SARS), and COVID-19.

Nipah
Nipah virus is part of the Paramyxovirus family, which also includes Hendra virus, Measles, Mumps. Nipah virus is carried by fruit bats. So far, Nipah outbreaks have been confined to South and Southeast Asia, but fruit bats are found in a large geographical area across the globe covering a population of more than 2 billion people.
Rift Valley Fever
Rift Valley Fever is an acute viral haemorrhagic disease caused by the Rift Valley Fever virus, belonging to the Phenuivirus family. This virus is transmitted by mosquitoes and blood feeding flies and most commonly affects domesticated animals but can also cause illness in people.