Pearl River, NY, USA, Seattle, WA and Oslo, Norway, March 13, 2020 — A Phase 1 clinical study of a Nipah virus vaccine candidate (HeV-sG-V) is underway. This trial marks the first time a vaccine developed to prevent Nipah virus infection will be studied in humans. No vaccines currently exist to protect against the deadly virus and the only treatment available is supportive care. CEPI first announced up to $25 million of funding for this programme in May, 2018.
The Phase 1 trial is a randomised, placebo-controlled, dose-finding study to investigate the safety, tolerability and immunogenicity of HeV-sG-V Nipah vaccine candidate. The study, sponsored by Auro Vaccines LLC and led by PATH, is being conducted at the Cincinnati Children's Hospital Medical Center in Cincinnati, USA. More information about the trial can be found using the ClinicalTrials.gov Identifier, NCT04199169.
The HeV-sG-V Nipah vaccine candidate
The Nipah virus vaccine programme is the result of a global partnership between Auro Vaccines, PATH and CEPI. The vaccine was originally developed by Dr. Christopher Broder and Dr. Katharine Bossart at the US government's Uniformed Services University of the Health Sciences (USU, Bethesda, MD). The HeV-sG-V Nipah vaccine candidate is a recombinant subunit vaccine that contains a portion of the G glycoprotein of Hendra virus, a henipavirus closely related to Nipah.
HeV-sG-V has been shown in preclinical studies to protect against Nipah virus and Hendra virus and has been licensed to Auro Vaccines from The Henry M. Jackson Foundation for the Advancement of Military Medicine, (HJF) through the USU/HJF Joint Office of Technology Transfer, to develop the human vaccine. Auro Vaccines, with the support of CEPI, will be sponsoring the Phase 1 study; PATH, an international global health organisation, is leading clinical operations; and CEPI is funding the programme through Phase 2 clinical study. Under the terms of the Framework Partnering Agreement originally awarded by CEPI for the collaboration among the three parties, Emergent BioSolutions Inc. has provided contract development and manufacturing services out of its Gaithersburg and Baltimore facilities in Maryland to produce the Phase 1 clinical trial material. Emergent, through a separate agreement with Auro Vaccines, has an exclusive option to license and to assume control of development activities for the Nipah virus vaccine candidate.
Auro Vaccines is committed to developing and delivering effective viral vaccines to address unmet medical needs. Our partnership with CEPI is evidence of the robustness of our vaccines technological platform. This development is another milestone towards our strategy of building a broad vaccine portfolio.
With a WHO-estimated case fatality rate of 40-75%, outbreaks predominantly in low- and middle-income countries, and no treatment or vaccine, PATH's Center for Vaccine Innovation and Access is keen to be a partner in the accelerated development of a Nipah vaccine. We look forward to advancing the first clinical trial of a Nipah virus vaccine candidate, and, if the evidence supports doing so, determining in Phase 2 trials the potential of this vaccine candidate to protect against and stop deadly Nipah virus outbreaks.
Emergent BioSolutions is pleased to collaborate with Auro Vaccines, CEPI, and PATH to support bringing the first Nipah vaccine candidate to the clinic. We are grateful for the opportunity to leverage our molecule-to-market contract development and manufacturing (CDMO) services and tap into the expertise of our Gaithersburg and Baltimore Bayview operations in Maryland to provide broad development service and drug substance capabilities, capacities, and expertise in synergy with our partners as we worked towards the same goal.
CEPI currently has four early-stage Nipah vaccine candidates in our portfolio and this will be the first to reach phase 1. This trial is an important first step toward making an effective vaccine available to at-risk populations in order to protect them from this deadly emerging infectious disease.
About Nipah virus
Nipah is a zoonotic disease, meaning it passes from animals to humans. The natural hosts of the virus are fruit bats. Nipah virus can be spread to people from infected bats, infected pigs, or infected people. Nipah virus infection can cause severe, rapidly progressive illness that affects the respiratory system and the central nervous system, including inflammation of the brain (encephalitis). Nipah virus has the potential to cause widespread illness and death and is included in the World Health Organization's 2018 R&D Blueprint list of priority diseases. Nipah virus was first identified in Malaysia in 1999 and has since been identified in Bangladesh and India as well, where repeated outbreaks have occurred as recently as February 2020. Many more countries throughout southeast Asia are at risk (WHO, 2020).
About CEPI
CEPI is an innovative partnership between public, private, philanthropic, and civil organisations, launched at Davos in 2017, to develop vaccines to stop future epidemics. CEPI has reached over US$750 million of its $1 billion funding target. CEPI's priority diseases include Ebola virus, Lassa virus, Middle East Respiratory Syndrome coronavirus, Nipah virus, Rift Valley Fever virus and Chikungunya virus. CEPI also invests in platform technologies that can be used for rapid vaccine and immunoprophylactic development against unknown pathogens (ie, Disease X). To date, CEPI has committed to investing over $480 million in vaccine and platform development. Learn more at www.cepi.net. Follow us at @CEPIvaccines.
About Auro Vaccines
Auro Vaccines LLC, a wholly owned indirect subsidiary of Aurobindo Pharma Ltd., is a company developing novel viral vaccines for the prevention and treatment of infectious diseases and related cancers.
Aurobindo Pharma Ltd. (NSE: AUROPHARMA, BSE: 524804, Reuters: ARBN.NS, Bloomberg: ARBP:IN), headquartered at Hyderabad, India, manufactures generic pharmaceuticals and active pharmaceutical ingredients. The company's manufacturing facilities are approved by several leading regulatory agencies like US FDA, UK MHRA, Japan PMDA, WHO, Health Canada, MCC South Africa, ANVISA Brazil. Aurobindo Pharma has several WHO prequalified products and is currently engaged in manufacturing Phase 3 materials of its 15-valent Pneumococcal conjugate vaccine in its state of art vaccine manufacturing facility in India.
With established strong market and manufacturing presence across the world in the areas of Oral Solids, Injectables, OTC & Dietary Supplement products and Branded Oncology business, the Company has set up strong capabilities in vaccines.
For more information, visit http://www.aurobindousa.com
About PATH
PATH is a global organization that works to accelerate health equity by bringing together public institutions, businesses, social enterprises, and investors to solve the world's most pressing health challenges. With expertise in science, health, economics, technology, advocacy, and dozens of other specialities, PATH develops and scales solutions—including vaccines, drugs, devices, diagnostics, and innovative approaches to strengthening health systems worldwide.For more information, visit http://www.path.org.
About Emergent BioSolutions, Inc.
As a global life sciences company whose mission is to protect and enhance life, we provide solutions that target public health threats. Through our specialty products and contract development and manufacturing services as well as our social responsibility efforts, we aspire to build healthier, safer communities and deliver peace of mind to our patients and customers so they can focus on what's most important in their lives. For more information visit www.emergentbiosolutions.com. Find us on LinkedIn and follow us on Twitter @emergentbiosolu and Instagram @life_at_emergent.
About the Uniformed Services University of the Health Sciences (USU)
The Uniformed Services University of the Health Sciences (USU), founded by an act of Congress in 1972, is the academic heart of the Military Health System. USU students are primarily active-duty uniformed officers in the Army, Navy, Air Force and Public Health Service who receive specialized education in tropical and infectious diseases, TBI and PTSD, disaster response and humanitarian assistance, global health, acute trauma care, and advanced practice nursing and dentistry. For more information, visit http://www.usuhs.edu.
About HJF
The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF) is a private, not-for-profit organization authorized by Congress to support military medicine at the Uniformed Services University of the Health Sciences and throughout the armed forces. HJF serves military, medical, academic and government clients by administering, managing and supporting preeminent scientific programs that benefit members of the armed forces and civilians alike. Since its founding in 1983, HJF has served as a vital link between the military medical community and its federal and private partners. HJF's support and administrative capabilities allow military medical researchers and clinicians to maintain their scientific focus and to accomplish their research goals effectively and efficiently. For more information, visit http://www.hjf.org.
Contact
CEPI:
Rachel Grant, Director of Communications and Advocacy
Phone: +44 (0) 7891249190
Email: [email protected]
Mario Christodoulou, Communications and Advocacy Manager
Phone: +44 (0) 7979300222
Email: [email protected]
Auro Vaccines:
Jeff Meshulam
Phone: +1 (443)224-8126
Email: [email protected]
PATH:
Lindsay Bosslet, Head of Communications and Public Relations
Phone: +1 (206)285-350
Email: [email protected]
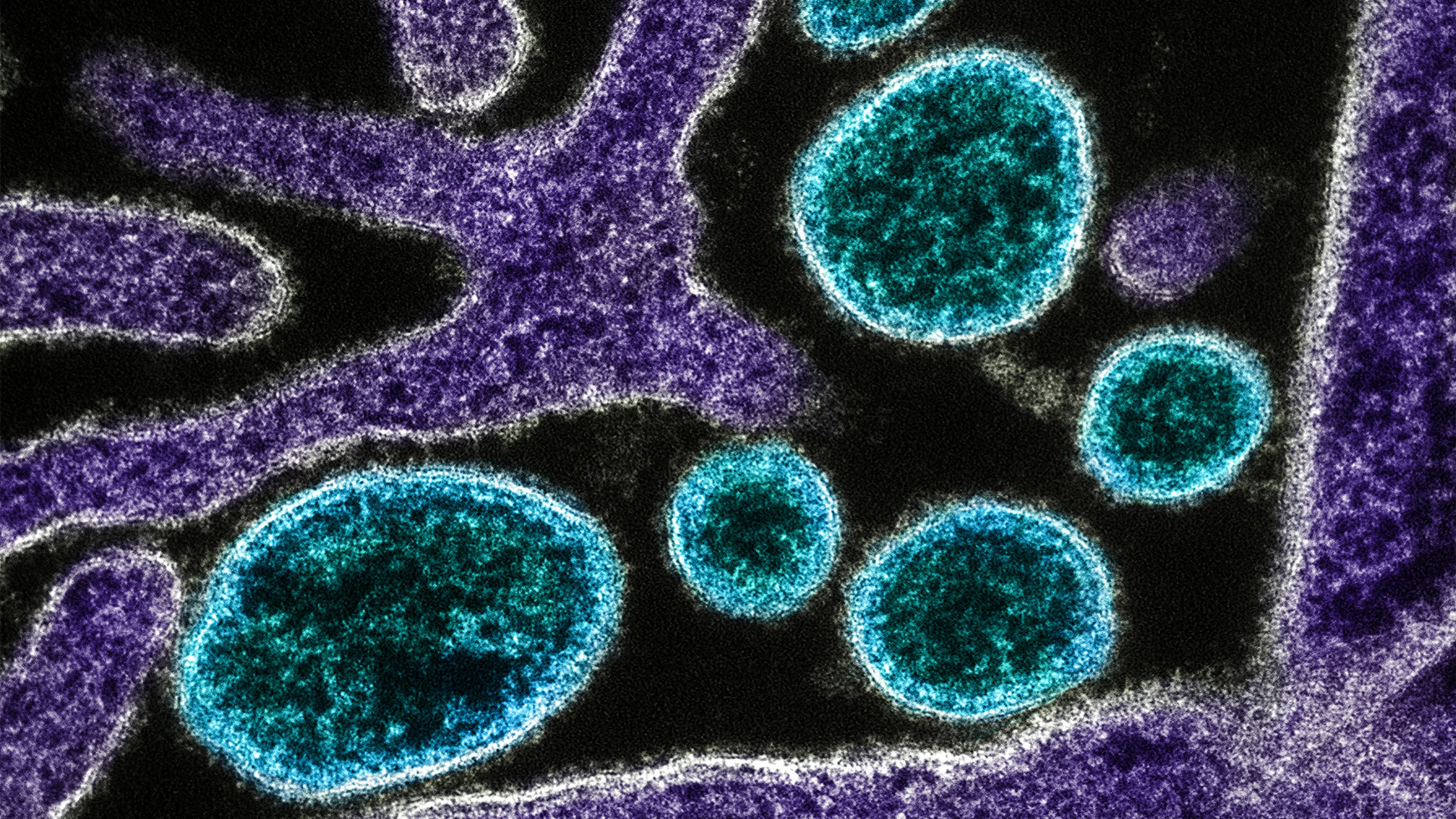
CAPTION AND CREDIT: Colorized transmission electron micrograph of mature extracellular Nipah Virus particles (blue) near the periphery of an infected VERO cell (purple). Image captured and color-enhanced at the NIAID Integrated Research Facility in Fort Detrick, Maryland. Credit NIAID


.webp)