- First-of-its kind trial will assess the safety and immunogenicity of theMVA-BN® vaccine in pregnant and breastfeeding women, and infants under two years of age.
- Research data could help to expand vaccine access to these priority populations, known to be at risk of severe complications from mpox but not currently eligible for vaccination.
- Participants will be enrolled into the clinical trial in Boende, DRC, from early 2025.
OSLO/BRUSSELS/ BOENDE, DRC, 7 Nov—A new scientific trial evaluating Bavarian Nordic’s mpox vaccine in pregnant and breastfeeding women and infants is set to launch in the Democratic Republic of Congo (DRC), a country at the epicentre of the fast-spreading outbreak declared both a continental and global public health emergency.
Data generated by the pioneering research could expand access to Bavarian Nordic’s MVA-BN mpox vaccine—currently the only WHO prequalified vaccine for use in healthy adults and adolescents—to also include pregnant or breastfeeding mothers and infants under two years of age. This is vitally important as these priority populations are at heightened risk of severe complications following mpox infection but are not currently eligible to receive a vaccination.
Around 350 pregnant women and 250 children aged two or under are due to take part in the study which will assess the safety and immunogenicity of the MVA-BN® vaccine in these populations for the first time. The trial will take place in Boende, DRC, beginning in early 2025.
The USD 8.1 million trial is jointly funded by the Coalition for Epidemic Preparedness Innovations (CEPI), providing USD 6.4 million, and Global Health EDCTP3, providing USD 1.7 million. Global Health EDCTP3 awarded its funding following the launch of an Emergency Call in May 2024, and the project kicked off in August 2024 as endeavours to mobilise additional support continued, culminating in the partnership between the two funders.
The project includes the University of Antwerp (Belgium) as Trial Sponsor, the University of Kinshasa (DRC) as Scientific Project Leader, and academic and research organisations Penta (Italy) and ACE Research (Kenya) as trial partners. Bavarian Nordic will supply the MVA-BN vaccine doses.
“Pregnant women and infants are particularly vulnerable to mpox, but their access to the MVA-BN vaccine will be limited until there is more data about its safety and immunogenicity in these populations” said Dr Nicole Lurie, Executive Director of Preparedness and Response at CEPI.
“I am thrilled that we are working with Global Health EDCTP3 and an international group of partners to make a vital contribution to protecting those most at risk of this devastating disease – both for those affected now and for all pregnant women and infants who may face the threat of mpox in the future.”
The current outbreak escalating in the DRC and neighbouring nations is the deadliest on record, with over 37,500 suspected cases and 1040 fatalities reported this year alone, many of them among children.
Dr Michael Makanga, Executive Director of Global Health EDCTP3, said: “Global Health EDCTP3 has been at the forefront of the Mpox outbreak response, with rapid mobilisation of funding through our emergency call. It enabled the trial (PregInPoxVac) to commence activities prior to the PHEIC declaration. Addressing major unmet needs of underserved groups is at the core of the work we fund, and I am delighted that we have partnered with CEPI to improve Mpox vaccine accessibility for the most vulnerable populations and achieve greater impact in our collective response to this crisis.”
The research will run as a randomised controlled trial with participants followed up over a twelve-month period. In the first stage of the study, two doses of the MVA-BN® vaccine will be administered to pregnant women before or after birth. Blood samples and breastmilk will be collected from a group of mothers and their infants to assess whether maternal antibodies are passed onto the newborn following vaccination via either of these routes.
In the trial’s second stage, infants aged 6-24 months old will receive a full or half dose or the MVA-BN® vaccine.
Data from the trial will be published in open-access journals so that it can be readily available to the entire public health community to inform current outbreak response efforts and future research.
Professor Jean-Pierre Van Geertruyden, Head of the Global Health Institute at the University of Antwerp, trial sponsor, said: “Though we expect the MVA-BN® vaccine to be efficacious and safe , there are limited data available in the target groups prohibiting licensure. Additionally, infants and pregnant women are easy to reach via well-established routine programmes, such as the Expanded Programme on Immunisation (EPI) and antenatal consultations.”
Professor Hypolite Muhindo Mavoko, University of Kinshasa, Scientific Project Leader and trial partner, said: “This trial marks an essential step towards closing the gap on vaccination of some vulnerable populations: pregnant women and infants. This offers an opportunity not only to address a pressing health need but also aiming to strengthen local research capacities to respond to future health crises. By enrolling approximately 350 pregnant women and 250 infants, the study promises essential insights that could expand vaccine options for the benefit of vulnerable populations worldwide.”
H.E Dr. Jean Kaseya, Director General of Africa CDC, said: "Protecting our most vulnerable populations—pregnant women and infants—has always been at the heart of Africa CDC’s mission. This groundbreaking study in the DRC marks a major step forward in mpox prevention for those at greatest risk. By generating critical data on the safety and effectiveness of the MVA-BN vaccine in these priority groups, we are paving the way for equitable vaccine access and bolstering our collective defense against this devastating disease. Africa CDC remains steadfast in its commitment to supporting innovations that safeguard the health and well-being of every community across the continent."
Paul Chaplin, President and Chief Executive Officer of Bavarian Nordic, said: “We applaud CEPI and their partners for enabling this trial, which will provide important data and learnings about the use of our non-replicating MVA-BN vaccine in pregnant women and infants, who remain highly vulnerable to mpox infections. This will complement other ongoing studies, also supported by CEPI, aiming to expand access to MVA-BN for priority populations, who are contraindicated to replicating vaccines.”
The trial is one of five projects Global Health EDCTP3 announced as part of efforts to address some of the most pressing challenges posed by the current outbreak. Global Health EDCTP3 is supporting another trial (PREGMPOX) assessing the impact of Mpox on pregnant women and newborns’ health.
CEPI is already supporting two other trials of Bavarian Nordic’s MVA-BN® mpox vaccine in Africa. These studies will generate evidence about the vaccine in African populations to help inform vaccination strategies and accelerate additional regulatory approval of MVA-BN® in endemic countries.
ENDS
This press release is also available in French.
Notes to Editors
- Research indicates that pregnant women are at an increased risk of severe illness and disease-related complications such as adverse foetal outcomes due to transmission of the mpox virus from mother to foetus during pregnancy. Similarly, infants are more likely to experience severe mpox disease manifestations, which underscores the importance of targeted public health interventions and vigilant monitoring to mitigate these risks.
- Mpox was first identified in the DRC in 1970. In most cases, mpox symptoms—typically fever and headache, followed by painful lesions—disappear within a few weeks. However, for some, mpox can lead to medical complications, such as bronchopneumonia, sepsis, encephalitis, loss of vision, and even death. The current mpox outbreak is being caused by two subgroups of the Clade I strain: Clade Ia and Ib. The current Clade Ib strain is causing heightened alarm because of the speed with which it has spread and potential changes to transmission dynamics.
About MVA-BN
MVA-BN or Modified Vaccinia Ankara-Bavarian Nordic is the only non-replicating mpox vaccine approved in the U.S., Switzerland, Singapore and Mexico (marketed as JYNNEOS®), Canada (marketed as IMVAMUNE®), and the EU/EAA and United Kingdom (marketed as IMVANEX®). Originally developed as a smallpox vaccine in collaboration with the U.S. government to ensure the supply of a smallpox vaccine for the entire population, including immunocompromised individuals who are not recommended vaccination with traditional replicating smallpox vaccines, MVA-BN has been indicated for use in the general population (from 12 years old) in individuals considered at risk for smallpox or mpox infection.
About CEPI
CEPI was launched in 2017 as an innovative partnership between public, private, philanthropic and civil organisations. Its mission is to accelerate the development of vaccines and other biologic countermeasures against epidemic and pandemic threats so they can be accessible to all people in need. CEPI has supported the development of more than 50 vaccine candidates or platform technologies against multiple known high-risk pathogens or a future Disease X. Central to CEPI’s pandemic-beating five-year plan for 2022-2026 is the ‘100 Days Mission’ to compress the time taken to develop safe, effective, globally accessible vaccines against new threats to just 100 days.
About Global Health EDCTP3
Established in 2021, Global Health EDCTP3 is a partnership between the European Commission, representing the European Union, and the EDCTP Association, representing the governments of 15 European and 29 sub-Saharan African countries. It builds on the work initiated by the European & Developing Countries Clinical Trials Partnerships (EDCTP) since 2003.
Global Health EDCTP3 funds collaborative clinical research on medical interventions to prevent, detect, treat and track infectious diseases prevalent in Africa. It also supports the strengthening of research capacities in sub-Saharan African countries to conduct high quality clinical studies and to prepare for and respond to (re-)emerging infectious diseases.
In response to the Mpox outbreak, Global EDCTP3 mobilised emergency funding and launched a dedicated call for proposals on 30 April 2024 which resulted in the funding of initially five research projects tackling Mpox, with additional funding for four reserve awards to be announced soon. The projects are supported with funds from the European Union, the Department of Health and Social Care of the United Kingdom and ANRS Maladies infectieuses émergentes (MIE).
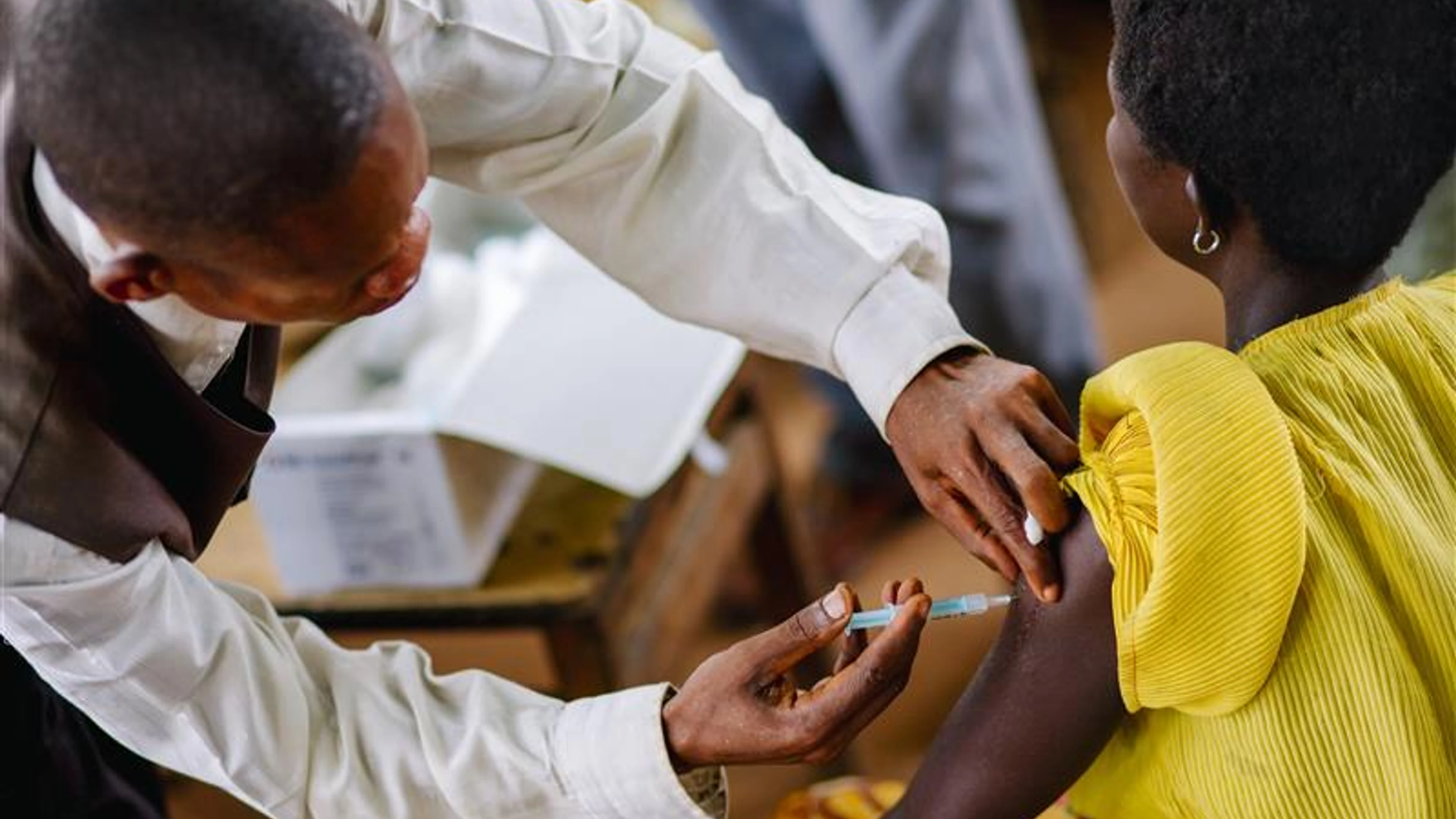
Photo Caption and Credit: Doctor from UNICEF mission doing the tetanus vaccination-DRC September 2008.


.webp)
