Calls for Proposals
Our Calls for Proposals invite innovators worldwide to apply to our scientific programmes to advance the development and manufacture of vaccines and tools against epidemic and pandemic threats. Learn more about our Open Calls and how to apply below.

What CEPI funds
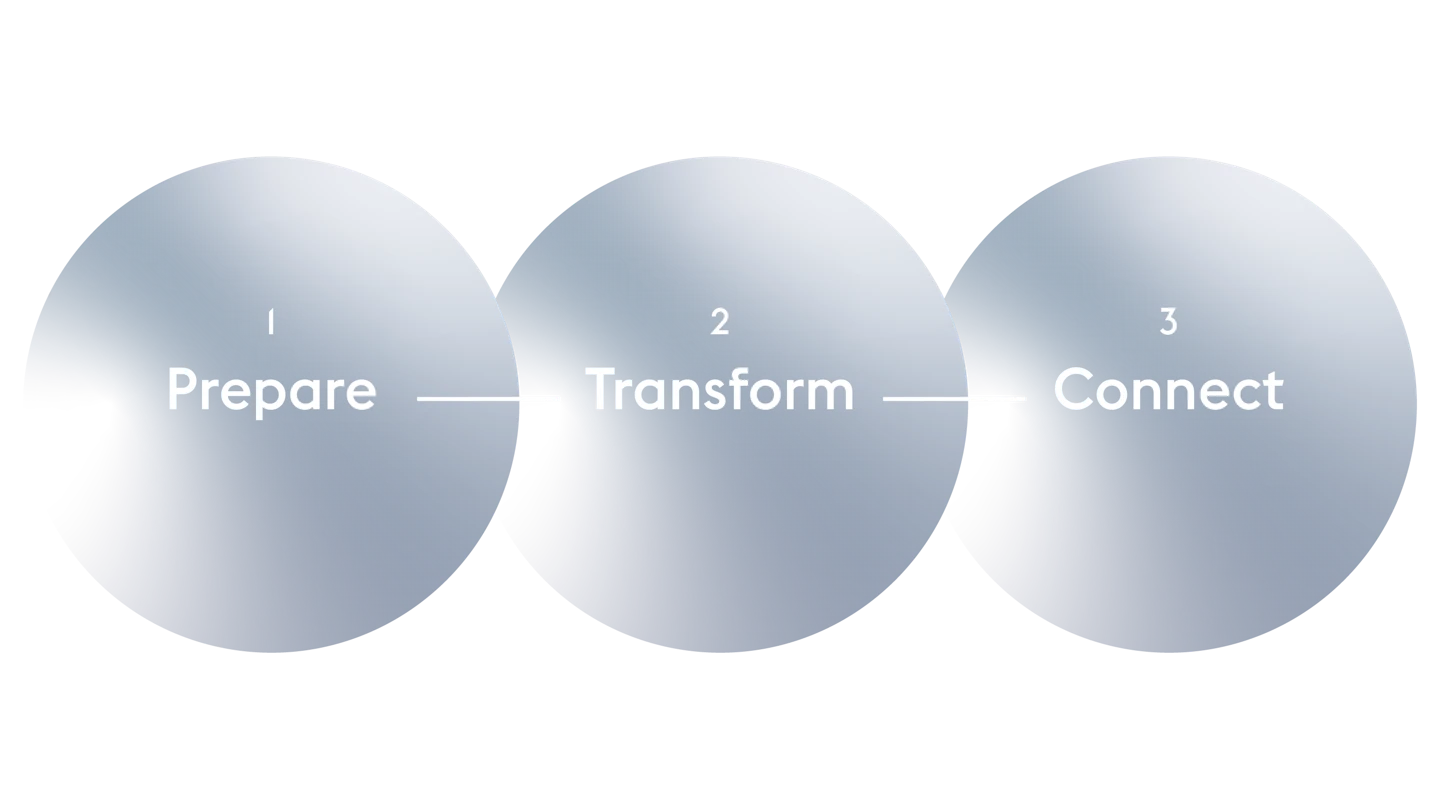
CEPI’s pandemic plan aims to substantially reduce or even eliminate the future risk of pandemics and epidemics.
The plan is set around three pillars: prepare for known threats; transform the response to the next novel outbreak; and connect and enhance global collaboration to strengthen global preparedness.
Explore our Calls for Proposals
Find out more about our Open Calls and details on how to apply below. You can also explore our archive of previous funding calls.
Open Calls for Proposals
Vaccine development and manufacturing
Enabling science
CEPI Tech Talks: tell us about your technology and approach
We are interested in finding out more about innovative technologies and projects you may be working on which may be relevant for later CEPI Calls.
If you would like to get a broader understanding of a CEPI Call for Proposal and/or see if your idea, project, technology, or approach meets our suitability and eligibility requirements, ahead of filling out your application or getting involved with CEPI, we can organise a “CEPI Tech Talk”.
CEPI Tech Talks are video calls of up to 50 minutes where you are invited to present your idea, project, technology, or approach and ask questions about its relevance to our Open Calls. We’ll also provide you with some guidance and recommendations on your project and a potential application to one or more of CEPI’s Calls.
We are particularly interested in hearing from vaccine developers and researchers in the Global South. CEPI will prioritise ideas that we perceive to best meet our suitability and eligibility requirements.
Arrange a CEPI TechTalk
If you’d like to arrange a CEPI TechTalk, email [email protected], with the subject line ‘CEPI Tech Talk: *Name* and *Organisation*’. Note the Call you are interested in, alongside a short description of your proposal/technology concept and any specific questions. Please also attach a non-confidential slide deck that best describes your idea, technology or approach.
Get in touch