Pandemic speed: How rapid-response science saves lives
CEPI stepped up quickly when a novel coronavirus emerged in China in late 2019 and became one of the first organisations to begin funding the development of vaccines. Within a few months. It had built one of the largest development portfolios of COVID-19 vaccines, seven of which have been licensed for international or domestic use.
CEPI’s leaders explain how speed, scale and access are essential to the 100 Days Mission which aims to accelerate the development of pandemic-busting vaccines.
___________
When Stephane Bancel, chief executive of the now well-known pharmaceutical firm Moderna, emailed CEPI on the evening of January 20, 2020, it was to ask for a million-dollar, potentially pandemic-busting decision.
Bancel, who had just arrived at the World Economic Forum in Davos in the Swiss Alps and had been closely watching the novel coronavirus that was starting to spread from Wuhan in China, was eager to push ahead with a scientific development he thought could become a world first and help protect against this worrying outbreak.
Even though the novel coronavirus hadn’t been around long enough even to earn its own name, Moderna’s scientists had already been hard at work on a totally new mRNA-based rapid response vaccine construct they thought might be able to protect against it. But they needed money—fast—to get clinical development of the experimental vaccine off the ground.
“We were anxious to get moving,” Bancel recalled afterwards. “We had known about the virus since between Christmas (2019) and New Year (2020), but without the genetic information we could do nothing. But then came a moment that we could start moving.”
For CEPI’s pandemic-watchers, it was a no-brainer. CEPI’s CEO Dr Richard Hatchett took just seven minutes to respond positively to Bancel’s email. And with catalytic funding of just under $1 million from CEPI, Moderna was able to get going on making its first test batches of experimental coronavirus vaccines.
“Starting early is the name of the game, because you can never make up for lost time,” says CEPI’s Executive Director of Preparedness and Response Dr Nicole Lurie. “We (at CEPI) had a deep appreciation of the need not just to move science fast, but to move money fast, and the need to be deadly serious about it.”

The seven-minute decision to fund Moderna was swiftly followed by two more lightning-fast CEPI investments—one with the American biotech company Inovio and the other with a team of vaccinologists at the University of Queensland in Australia.
This meant that by January 23, 2020 when just 581 cases of infection with SARS-CoV-2 had been confirmed worldwide, CEPI had already kick-started three vital investments into potential defences against it.
And it didn’t stop there. In the months that followed, CEPI built one of the world’s largest and most diverse COVID-19 vaccine portfolios, based on the principles of speed, scale and access.
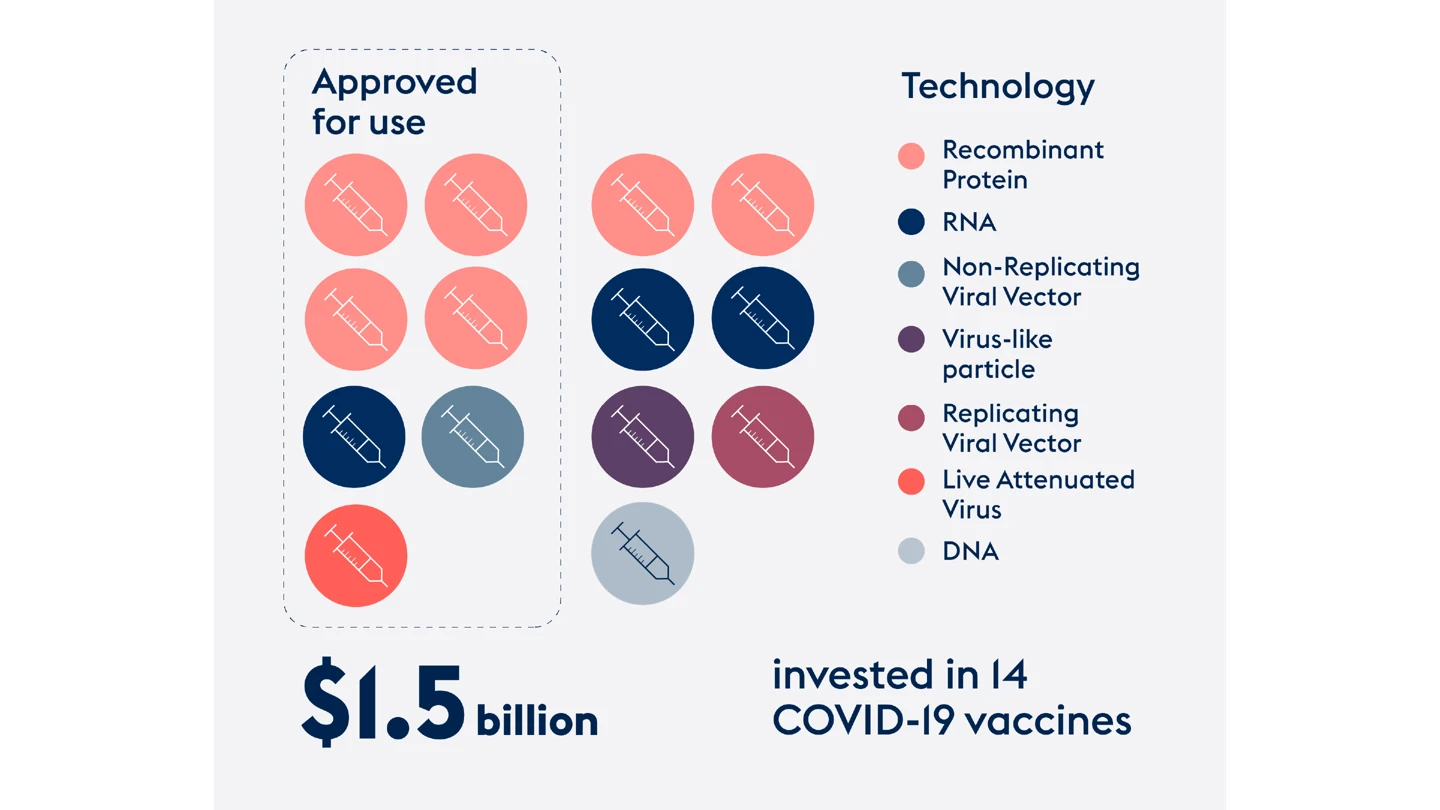
Ultimately CEPI invested more than $1.5 billion to advance the development of 14 different COVID-19 vaccines using a range of technologies from viral vector approaches to protein- and mRNA-based vaccines.
Today, the success ratio for those vaccines stands at 50 percent – with seven proving safe and effective and gaining regulatory approval for domestic or global use.
Figures vary from study to study, but it’s clear that the impact of those rapid response decisions has proved life-saving many, many times over.
Research has found that the deployment of multiple swiftly-developed safe and effective COVID vaccines—including Moderna's and the Oxford-AstraZeneca shot which CEPI also part-funded—helped prevent tens of millions of deaths around the world. One study found that COVID-19 vaccines collectively saved nearly 20 million lives in just their first year of rollout.
Through COVAX—the first global vaccine-sharing facility which CEPI conceptualised and co-led with the World Health Organization, UNICEF and GAVI—an estimated 2.7 million lives were saved in lower and middle-income countries thanks to the delivery of almost 2 billion doses of COVID-19 vaccines to 146 countries.
It was the recognition of the need for speed and equity of access, coupled with the recognition of the ground-breaking scientific advances that were made in vaccine development for COVID-19, that catalysed CEPI’s thinking about the 100 Days Mission—an ambitious initiative aiming to accelerate vaccine development timelines to around three months in the event of a looming global disease threat.
This new approach—designed to get ahead of viral disease outbreaks—would enable the world to swiftly counter emerging threats before they spiral into full-scale pandemics.
The 100 Days Mission’s focus is not only on speedy science and rapid scale-up, but also on fostering international collaboration in preparing for pandemics, so that when the next viral threat emerges, safe and effective vaccines can be deployed equitably and efficiently.
As CEO Dr Hatchett puts it: “If we are serious about responding effectively to future pandemic threats—if we even want to dream about preventing future pandemics—we must master the execution and mechanics of response.”
Which is why, by progressing and adding to just the kind of scientific advancements made during COVID-19, CEPI is mobilising governments, pharmaceutical companies and research institutions all over the world to invest in innovations that bring pandemic speed to preparedness and response. The hope is that ultimately, such foresight will stop future pandemics before they start.
$1.5 billion
invested to advance the development of 14 different COVID-19 vaccines
7
CEPI-backed COVID-19 vaccines approved for domestic or global use
~2 billion
doses delivered through COVAX



