In June, health leaders gathered in Johannesburg for the G20 Health Working Group meeting, as part of South Africa’s G20 Presidency to discuss critical global health issues. High on the agenda was the topic of pandemic prevention, preparedness, and response.
Building on the momentum from the adoption of thePandemic Agreement at the World Health Assembly in May 2025, I had the privilege of addressing this group to reflect on global progress, and gaps, in this crucial area of global health.
Five years on from the COVID-19 pandemic, we stand at a critical juncture. The threat of future pandemics has not faded, and the urgency remains.
The 100 Days Mission, first launched during the UK’s G7 presidency in 2021 through a partnership with CEPI and later endorsed by G20 leaders, offers a clear and actionable roadmap: within 100 days of identifying a viral threat with pandemic potential, the world must be ready to produce safe, effective, and affordable diagnostics, therapeutics, and vaccines (DTVs) at scale.
Potential impact of the 100 Days Mission on COVID-19
Had the 100 Days Mission been achieved in response to the COVID-19 pandemic, over 8 million lives could have been saved, according to a CEPI-backed modelling study done by researchers at Imperial College. In lower-middle income countries alone, it could have prevented nearly 800 million infections and over 15 million hospitalisations by the end of 2021.
But it is not simply about speed – it’s also about equity, access, and a shift in how we prepare our systems during the periods between pandemics. Diagnostics enable early detection and containment. Therapeutics reduce disease severity and save lives. Vaccines provide population-level protection. Together, they form the trifecta of our pandemic defence.
Urgent need to fill gaps in our global health security
However, the latest 100 Days Mission Scorecard, developed by International Pandemic Preparedness Secretariat (IPPS) and Impact Global Health, reveals sobering findings. R&D funding has declined sharply since 2022. Most of the 25 or so viral families known to affect people still lack a complete set of approved DTVs. Beyond COVID-19, the pipeline of DTVs is sparse and far too reliant on a single dominant R&D funder, the U.S. government, which provided 60% of all DTV funding between 2014 and 2023.
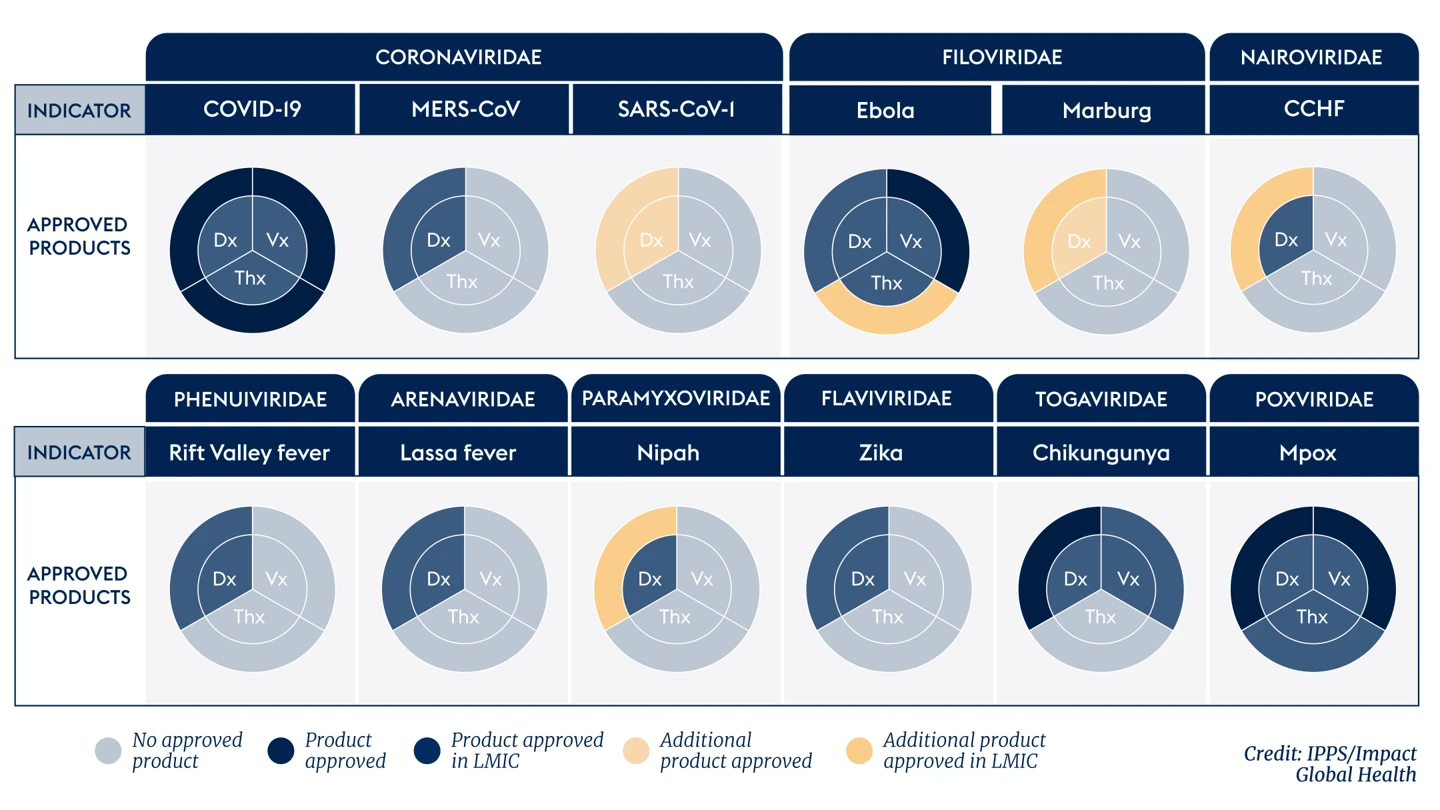
We also see systemic weaknesses: underuse of alternative regulatory pathways, limited translation of candidates from preclinical to clinical stages, and minimal progress on key enablers such as establishing trial infrastructure in low-income and middle-income countries and establishing correlates of protection for key pathogens.
To address these weaknesses, the world must diversify funding sources, strengthen R&D capabilities globally, and improve coordination of resource allocation and regulation. This is not a job for any one actor. It requires public-private partnerships, global institutions, national governments, and philanthropic organisations all working together.
Therapeutics in particular represent a critical and under-developed area. The Scorecard, in addition toanalysis by the INTREPID Alliance, show that the pipeline for therapeutics is bare, with minimal R&D activity across all non-COVID viral families and a 58% drop in funding in 2023 from 2022. This is an alarming picture, given how vital therapeutics are in reducing mortality and slowing disease progression – especially while vaccines are being developed, in cases where vaccine development is difficult or impossible, or where some sections of the population may be unwilling or unable to receive vaccines.
The case couldn’t be clearer: we need therapeutics now to work in unison with diagnostics and vaccines against the next outbreak.
To address this, IPPS has been working with partners to operationalise a Therapeutics Development Coalition—a global public-private partnership to reinvigorate the pipeline. This coalition would align public and private funding calls with known needs and ensure sustained investment throughout the development lifecycle. Its goals include developing at least two Phase II-ready therapeutics per priority viral family, increased support for platform technologies, pre-agreeing trial and manufacturing pathways, and long-term sustainable investment. It also aims to complement existing initiatives such as WHO’s Collaborative Open Research Consortiums and the i-MCM-net.
The 100 Days Mission is more than a scientific challenge—it is a strategic challenge. That is why IPPS is engaging with partners to ensure that pandemic tools reach those who need them, when they need them.
The G20 has a vital leadership role to play and as G20 Health Ministers prepare to gather in November, there are four critical steps they must consider to continue progress towards the 100 Days Mission: adopt scorecards like the one developed for the 100 Days Mission to monitor pandemic preparedness; commit to coordinated R&D investment that embeds equitable access principles; support innovative public-private partnerships to fill the gaps in R&D such as the nascent Therapeutics Development Coalition; and harmonise regulatory frameworks for swifter, safer emergency response.
The world cannot afford to wait until the next crisis. The tools to act are within reach. With political will and coordinated effort, we can make the 100 Days Mission a reality and in doing so, save millions of lives in the future.



.webp)
