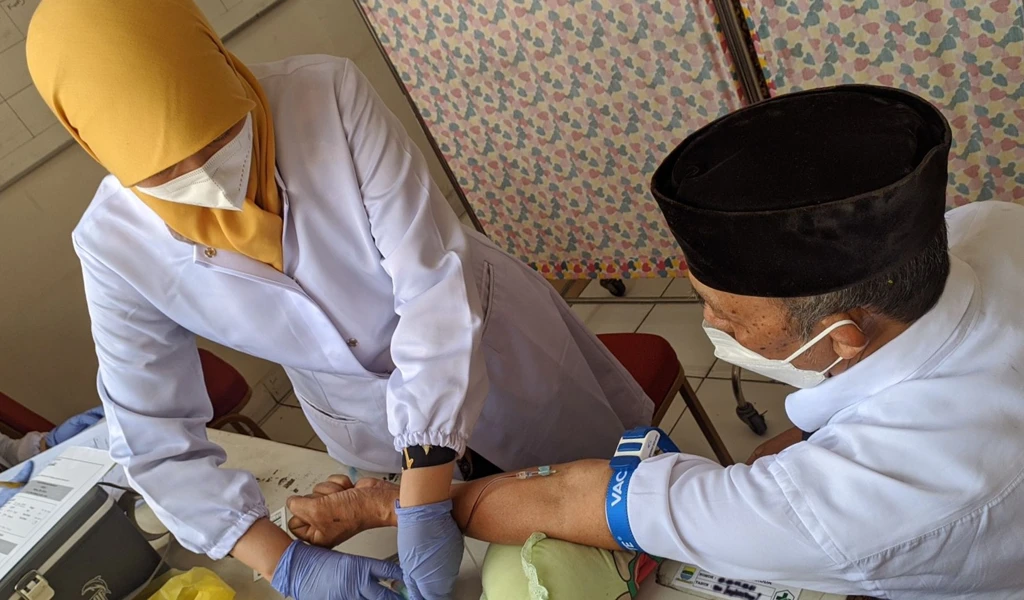
On 11 May 2020, CEPI announced an investment of up to $388 million to accelerate the development and manufacturing of Novavax' NVX‑CoV2373 vaccine candidate against COVID-19, rapidly advancing the vaccine candidate through clinical trials, while scaling up manufacturing capacity in parallel.
The first participants were today enrolled into a Phase 1/2 clinical trial of NVAX-CoV2373 which is being conducted at sites in Melbourne and Brisbane, Australia. Preliminary immunogenicity and safety results from the Phase 1 portion of the trial are expected in July 2020.
The Novavax vaccine candidate is the fourth CEPI-funded vaccine to enter clinical trials, along with Inovio's INO-4800 DNA vaccine candidate, Moderna's mRNA-1273 candidate, and the University of Oxford's ChAdOx1 nCoV-19 candidate.
Entering clinical trials is an important step on the path to delivering a safe, effective and globally accessible vaccine against COVID-19. Vaccines provide our best hope of permanently defeating this pandemic, so it is encouraging to see rapid progress being made in the development of Novavax' vaccine candidate.
CEPI's priority in building our portfolio has been to focus on vaccine candidates with the potential to be developed at speed and scale and made globally accessible. Our investment in Novavax allows us to focus on manufacturing in parallel with the clinical development of the vaccine, so that if the vaccine is proven to be safe and effective, we can make doses available to those who need them without delay.
The Phase 1/2 clinical trial is being conducted in two parts. The Phase 1 portion is a randomized, observer-blinded, placebo-controlled trial designed to evaluate the immunogenicity and safety of NVX‑CoV2373, both adjuvanted with Matrix‑M and unadjuvanted. The trial is enrolling approximately 130 healthy participants aged 18 to 59 years at two sites in Australia. If it is successful, the Phase 2 portion of the study is expected to be conducted in multiple countries, and will assess safety and immunogenicity in a broader age range.
It is anticipated that vaccines produced under this partnership will be procured and allocated through global mechanisms now under discussion as part of the Access to COVID-19 Tools (ACT) Accelerator, an international initiative launched by the WHO and global leaders in April 2020.
Image caption and credit: Vaccine vial hypodermic injection. Numstocker/ Shutterstock