In 2018, CEPI provided up to $19 million to Oxford to develop vaccines against Lassa, Nipah, and MERS.
In view of the global threat of COVID-19, CEPI expanded its collaboration with Oxford in March to use their ChAdOx1 viral vector technology to develop a vaccine candidate against the coronavirus. CEPI provided initial funding for the Oxford project to support the manufacture of vaccine materials required for preclinical and phase 1 testing.
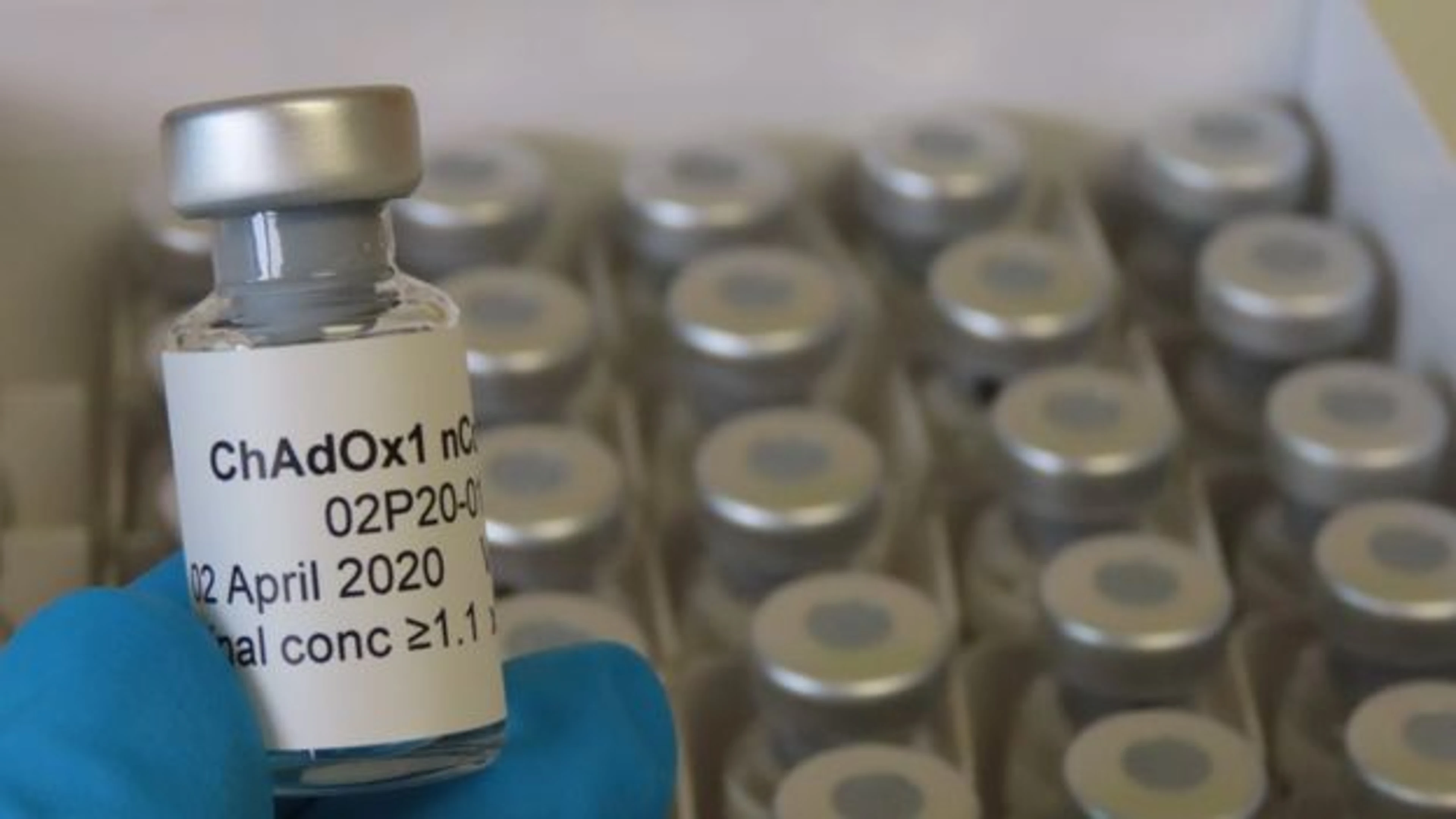
Now, just 6 weeks on, Oxford have begun human trials of their ChAdOx1 nCoV-19 vaccine candidate. The Oxford vaccine will be the third CEPI-funded vaccine to enter into phase 1 trials, along with Inovio's INO-4800 DNA vaccine candidate and Moderna's mRNA-1273 candidate.
We are pleased to see the rapid advancement of Oxford University's vaccine candidate into clinical safety testing. Producing a safe, effective, and globally accessible vaccine is our best hope in ending this pandemic. We aim to do this at a speed never before seen in vaccine development. To achieve this goal, unprecedented levels of collaboration and investment across private and public sectors will be needed. Oxford team have taken a significant step forward in that journey, but we still have a long road to travel.
The University of Oxford (ChAdOx1 vectored vaccine)
ChAdOx1 is a replication-deficient simian adenoviral vaccine vector. This vaccine platform has been used to produce vaccine candidates against multiple pathogens, including Influenza, Chikungunya, and Zika. Oxford's ChAdOx1 MERS candidate has completed phase 1 studies and a second clinical study is underway in Saudi Arabia.
Progress towards a COVID-19 vaccine
CEPI has worked at unprecedented speed to initiate eight COVID-19 vaccine development projects with Curevac, Inovio Pharmaceuticals, Moderna, Novavax, The University of Hong Kong, The University of Oxford, The University of Queensland and a consortium led by Institut Pasteur. The first clinical trials of CEPI-supported vaccines are already underway, and CEPI's ambition is to have at least three vaccine candidates which could be submitted to regulatory authorities for licensure for general use.
Find out more about the ChAdOx1 nCoV-19 vaccine trial here.
Image credit: Sean Elias