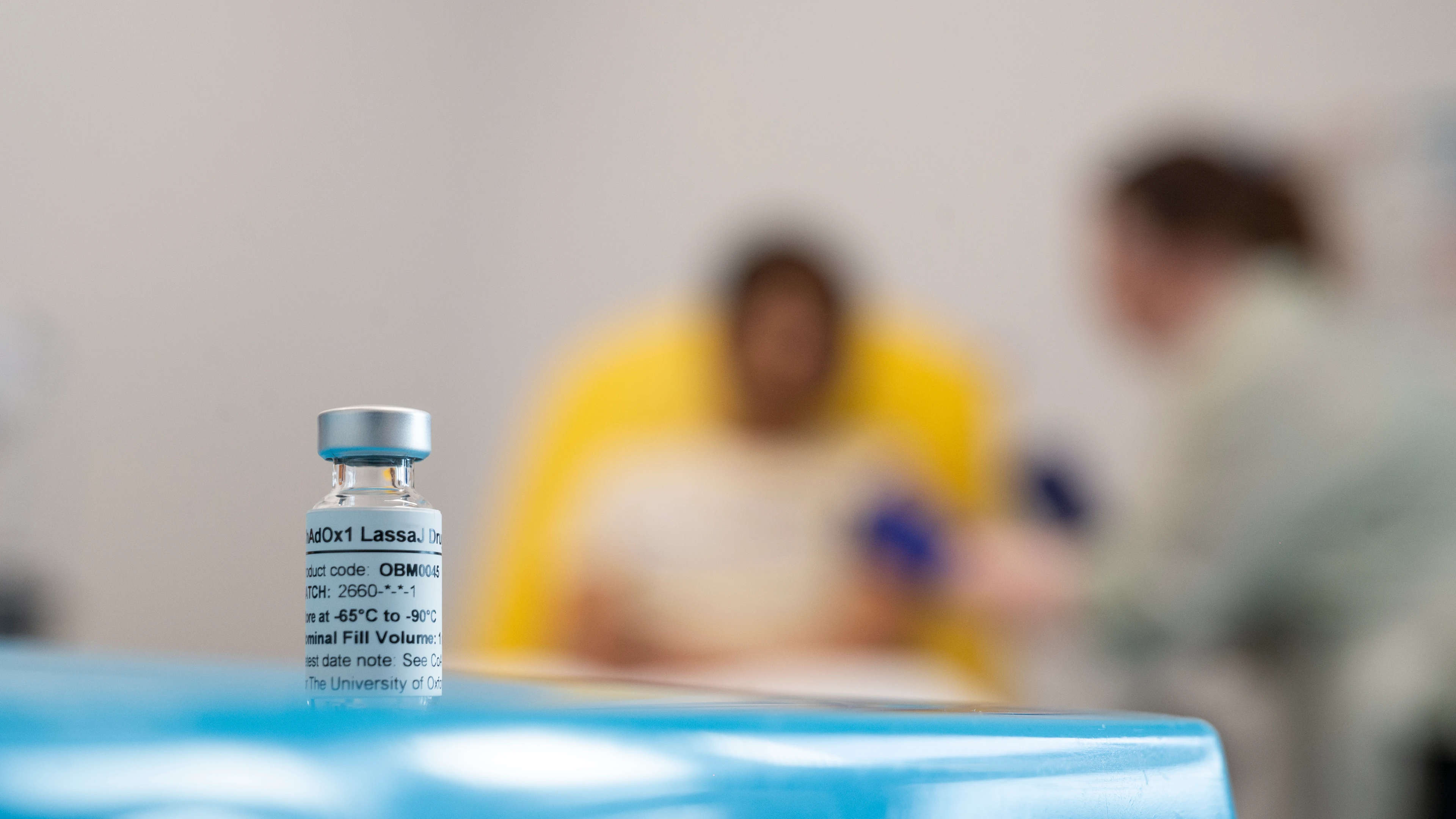
- The Oxford Vaccine Group has vaccinated the first volunteerin a first-in-human trial of its Lassa vaccine
- The CEPI-funded trial is being conducted in Oxford, with a second phase 1 trial due to start in Ghana early next year
- The World Health Organization (WHO) has identified Lassa fever as a priority pathogen in urgent need of research and development because it poses a significant public health risk due to its epidemic potential.
The first volunteer has received a dose in a first-in-human trial of Oxford’s Lassa vaccine, marking a major milestone in the fight against the deadly virus.
The trial, conducted by the Oxford Vaccine Group, and funded by the Coalition for Epidemic Preparedness Innovations (CEPI), will assess the safety and immune response of the ChAdOx1 Lassa vaccine. 31 people aged 18-55 will participate in the trial in total.
Lassa fever is caused by the Lassa virus, which is primarily spread by rodents and can result in serious illness including deafness, severe bleeding and even death. First discovered in the late 1960s in Nigeria, Lassa fever is endemic in West Africa. The World Health Organization (WHO) has identified Lassa fever and related viruses as priority pathogens in urgent need of research and development because they pose a significant public health risk due to their potential to cause large outbreaks.
Experts estimate that up to 700 million people could live in regions at risk of Lassa fever by 2070, although there are currently no licensed vaccines or treatments for Lassa fever.
Developed by researchers at the Pandemic Sciences Institute, University of Oxford, the vaccine is made using the same viral vector platform as the Oxford/AstraZeneca COVID-19 vaccine, which is estimated to have saved 6 million lives in its first year alone.
Commenting on the launch of the trial, Professor Maheshi Ramasamy, Chief Investigator of the trial at the Oxford Vaccine Group, said:
“Vaccines are one of the most powerful tools we have in global health: they save lives, stop outbreaks, and strengthen health systems, and so we’re delighted to start the VITAL01 Lassa fever vaccine study here in Oxford. Building on Oxford’s world-leading experience in developing vaccines for emerging infections and pandemics, including COVID-19, this study is a crucial step toward protecting vulnerable communities from the devastating impact of Lassa fever.”
CEPI also supported early preclinical development of the vaccine.
Dr Katrin Ramsaeur, Lassa Disease Programme Lead at CEPI, said: “Today marks a transformative milestone in the fight against the deadly Lassa fever. The launch of this Lassa vaccine clinical study has been made possible by years of rigorous and innovative science, steadfast collaboration, and an unwavering commitment to global health. While there is still important work ahead, this moment brings us closer to a future where communities no longer live in fear of this devastating disease.”
In addition to the launch of new clinical trials, plans to advance a Lassa vaccine to licensure are progressing through regional leadership and coordination by the Lassa fever Coalition. The consortium led by the West African Health Organization (WAHO), with support from CEPI and partners, is made up of West African leaders and public health experts working with vaccine makers to accelerate the development and future equitable introduction of Lassa fever vaccines across the affected region.
Dr Virgil Lokossou, Director of Healthcare Services at WAHO said: “For more than half a century, Lassa fever has affected lives in West Africa — from our families and livelihoods to our hospitals and economies. Now, our region is taking bold steps to change that story. By working together with partners like the University of Oxford through the Lassa fever Coalition, we are leading the way in confronting this epidemic threat so that it no longer undermines our health and societies. Oxford’s vaccine candidate brings real promise for protection against this deadly disease and this clinical trial arrives at a moment when regional efforts to defeat Lassa fever are stronger than ever.”
ENDS
Notes to editors
For more information on Lassa fever, please visit the Vaccine Knowledge website: https://vaccineknowledge.ox.ac.uk/lassa
The Oxford Vaccine Group is part of the Department of Paediatrics at the University of Oxford. It led the rapid clinical development of vaccinations against COVID-19 in the pandemic and has made significant contributions to knowledge, supporting national and global policy on immunisation over three decades. OVG was founded in 1994 by Professor E. Richard Moxon. It is one of the world’s leading academic vaccine research teams, and has been led by Professor Sir Andrew Pollard since 2001. The OVG undertakes vaccine research spanning basic science and preclinical studies through to epidemiological studies, human challenge models and phase I-III clinical trials. Current research includes the study of vaccines for outbreak pathogens and pandemics, enteric pathogens, bacterial and viral respiratory infections, and use of human challenge models to accelerate vaccine development.
- The Pandemic Sciences Institute is an interdisciplinary research institute at the University of Oxford dedicated to confronting the challenge of epidemic and pandemic infectious diseases. We work with academia, industry and public health organisations across the world to create science-led innovations, accelerate understanding, and develop new diagnostics, treatments, vaccines and disease control tools. PSI is hosted by the University’s Nuffield Department of Medicine. www.psi.ox.ac.uk
The University of Oxford has been ranked number 1 in the Times Higher Education World University Rankings for the ninth year running, and number 3 in the QS World Rankings 2024. At the heart of this success are the twin-pillars of our ground-breaking research and innovation and our distinctive educational offer. Oxford is world-famous for research and teaching excellence and home to some of the most talented people from across the globe. Our work helps the lives of millions, solving real-world problems through a huge network of partnerships and collaborations. The breadth and interdisciplinary nature of our research alongside our personalised approach to teaching sparks imaginative and inventive insights and solutions.
- CEPI is an innovative partnership between public, private, philanthropic and civil organisations. Its mission is to accelerate the development of vaccines and other biologic countermeasures against epidemic and pandemic threats so they can be accessible to all people in need. CEPI has supported the development of more than 70 vaccine candidates or platform technologies against multiple known high-risk pathogens or a future Disease X. Central to CEPI’s pandemic-beating plan is the ‘100 Days Mission’ to accelerate the time taken to develop safe, effective, accessible vaccines against new threats to just 100 days. Learn more at CEPI.net.
This press release is also available to view in French.


.webp)