This article was updated on 6 October 2022, 6 December 2022, & 2 May 2023 to include updates to CEPI's broadly protective coronavirus vaccine portfolio
We keep hoping it's all over. But sadly, the race against the coronavirus is still very much on. And it's a long one. Thanks in large part to those pernicious and persistent variants, beating this devastating COVID-19 pandemic is more like an ultra-marathon than a sprint. To help humanity get ahead, scientists are now raising their game.
Despite record-breaking new vaccines that are now protecting tens of millions of people from severe illness and death from COVID-19, the sad reality is that since its emergence in late 2019, we have always been one step behind SARS-CoV-2. As we've seen with Delta, Omicron, then BA.4, BA.5 and others, each new variant and sub-variant has proved more transmissible than the last, and each is as capable as the last of evading some of the protection offered by current vaccines.
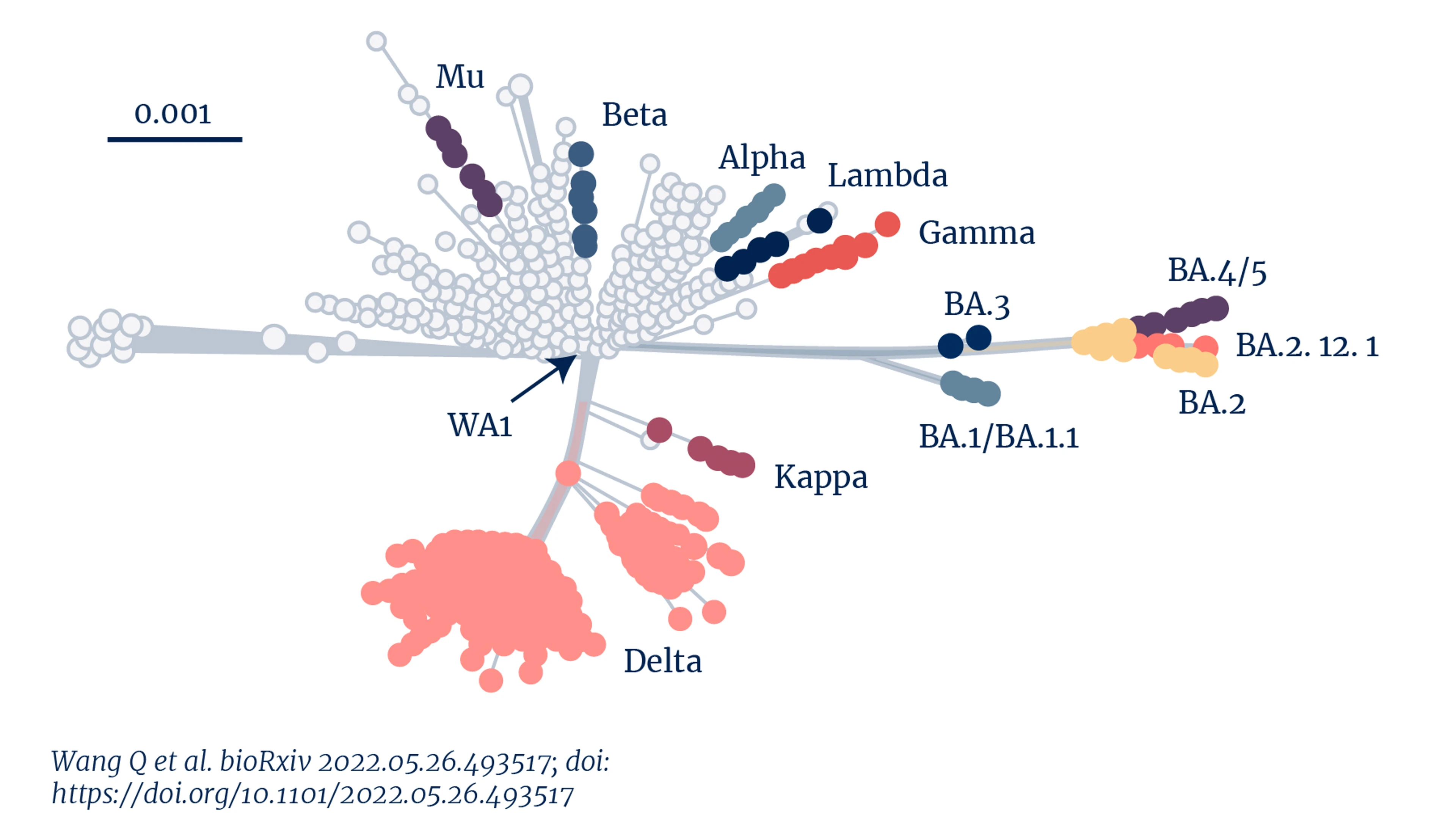
Genetic distance of SARS-CoV-2 variants. Credit: Wang Q et al. (2022)
"All of the COVID variants we've been seeing - the Greek alphabet of variants and their sub variants - have continued to evolve to be more highly transmissible each time. That shows us that, for now, we have an inability to get to grips with coronaviruses using the tools we currently have," says Christopher da Costa, programme leader for CEPI's next-generation coronavirus vaccine portfolio.
While we will soon have newly adapted COVID-19 vaccines that target Omicron, there's a high chance that by the time most people get access to them, they could already have been outrun by the next variant. All of this leaves us lagging behind, in a precarious ‘back of the pack' position. We are forever chasing SARS-CoV-2 and its variants, but never able to overtake them.
'Holy grail'
So, after setting new speed records in developing safe and effective first generation COVID-19 vaccines — the first ever vaccines against coronaviruses to be fully developed, approved and deployed — vaccine scientists are now in a new phase of the race. This time, their aim is to develop a next generation of tools that can put humanity ahead - not only of SARS-CoV-2 and its viral variants, but also of future potential coronavirus threats.
The ultimate prize at the end of this - the ‘holy grail' as some like to call it - is all-in-one, broadly protective, or "pan" coronavirus vaccines - shots that would pre-empt the evolution of new variants and sub-variants of COVID-19 and be a defence against future pandemic threats posed by novel coronaviruses that have yet to come across the viral frontier.
"The best way to truly be able to end this pandemic and prevent another coronavirus pandemic will be with vaccines that can protect us against future newly emerging coronaviruses and against new variants of this one. That's why CEPI is so focussed on this urgent and challenging work," says Melanie Saville, CEPI's Executive Director of Vaccine Research and Development.
New coronaviruses are bound to emerge
With the enormous loss of life and liberty brought about by the COVID-19 pandemic, we hardly need more evidence of the damage coronaviruses - both new and old - can cause. But if we did, the context of the last two decades helps underline the warning that as a viral family, coronaviruses are a deadly force to be reckoned with. COVID-19 is the third novel coronavirus to have made the jump from animals into humans since 2000. First came SARS-CoV-1, or Severe Acute Respiratory Syndrome (SARS), in 2003. Then, around a decade later, came MERS-CoV, the Middle East Respiratory Syndrome (MERS). Each of these coronaviruses caused major, deadly epidemics. Another decade on came COVID-19, unleashing a devastating pandemic on an unprepared world.
With three coronavirus epidemics or pandemics already in the 21st century alone, it's fair to say coronaviruses are right up there with flu as having dangerous pandemic potential.
And they're not going away. Surveillance studies have found that there are well over 100 coronaviruses circulating in animals that have the potential to spill over into humans. And a warming planet is only going to make things worse. Climate change, rapid urbanisation, and deforestation are all combining to drive up the risk of thousands of viruses jumping from one species of mammal to another — in turn upping the chances that one or more will cross over into people and cause a novel epidemic. So whether it's in another 10 years - or sooner, or later - it would be foolish to think another novel coronavirus won't someday make the same jump into humans that SARS, MERS and COVID-19 made. "With three coronavirus epidemics or pandemics already in the 21st century alone, it's fair to say coronaviruses are right up there with flu as having dangerous pandemic potential," says Saville. "Who knows when they will emerge, but there are bound to be more."
High risk, high reward
In vaccine R&D terms, designing and developing a variant-proof COVID-19 vaccine - or a broadly protective or pan-coronavirus vaccine - that will prove safe, effective and scalable is very much in the category of ‘high-risk, high-reward' work. The ‘high risk' element of that equation is largely due to the extreme complexity of the task. A safe and effective pan-coronavirus vaccine — something that hasn't been developed for flu despite decades of effort - would be a future-changing scientific and medical breakthrough. The ‘high reward' is the prospect of getting ahead of new variants of COVID-19 before they evolve, and, ultimately, of ensuring the world will never again be hit by a pandemic caused by a coronavirus.
Having recognised the wider coronavirus threat early, CEPI launched a call for proposals in March 2021 seeking potential developers of variant-proof COVID-19 vaccines, and broadly protective coronavirus vaccines. It has so far awarded around $230 million in grants to fund 13 separate projects — each of them exploring a distinct way of designing a shot that will be broadly protective against one or more coronaviruses. Since we don't know yet which scientific approaches will work, having a portfolio of options will maximise our chances of success — just as it did with first generation COVID-19 vaccine development. The $230 million investment makes CEPI by far the leading funder of research and development on vaccines that could prevent a future coronavirus pandemic. The next largest pot of research funding is from the U.S. National Institutes of Health, which has put up $36 million.
View CEPI's portfolio to date

CEPI's broadly protective coronavirus vaccine partners. Credit: CEPI.
For now, most research groups are focused on a subset of coronaviruses that have proved the most worrying so far — the Betacoronavirus group. It includes the original SARS-CoV-1, and MERS-CoV, as well as the pandemic SARS-CoV-2, some of the seasonal coronaviruses that cause common colds in people, and several other coronaviruses that circulate widely in bats. A subset of that subset, known as the sarbecoviruses, which excludes MERS but includes both the human SARS viruses as well as several SARS-like bat viruses, is the focus of other teams' work.
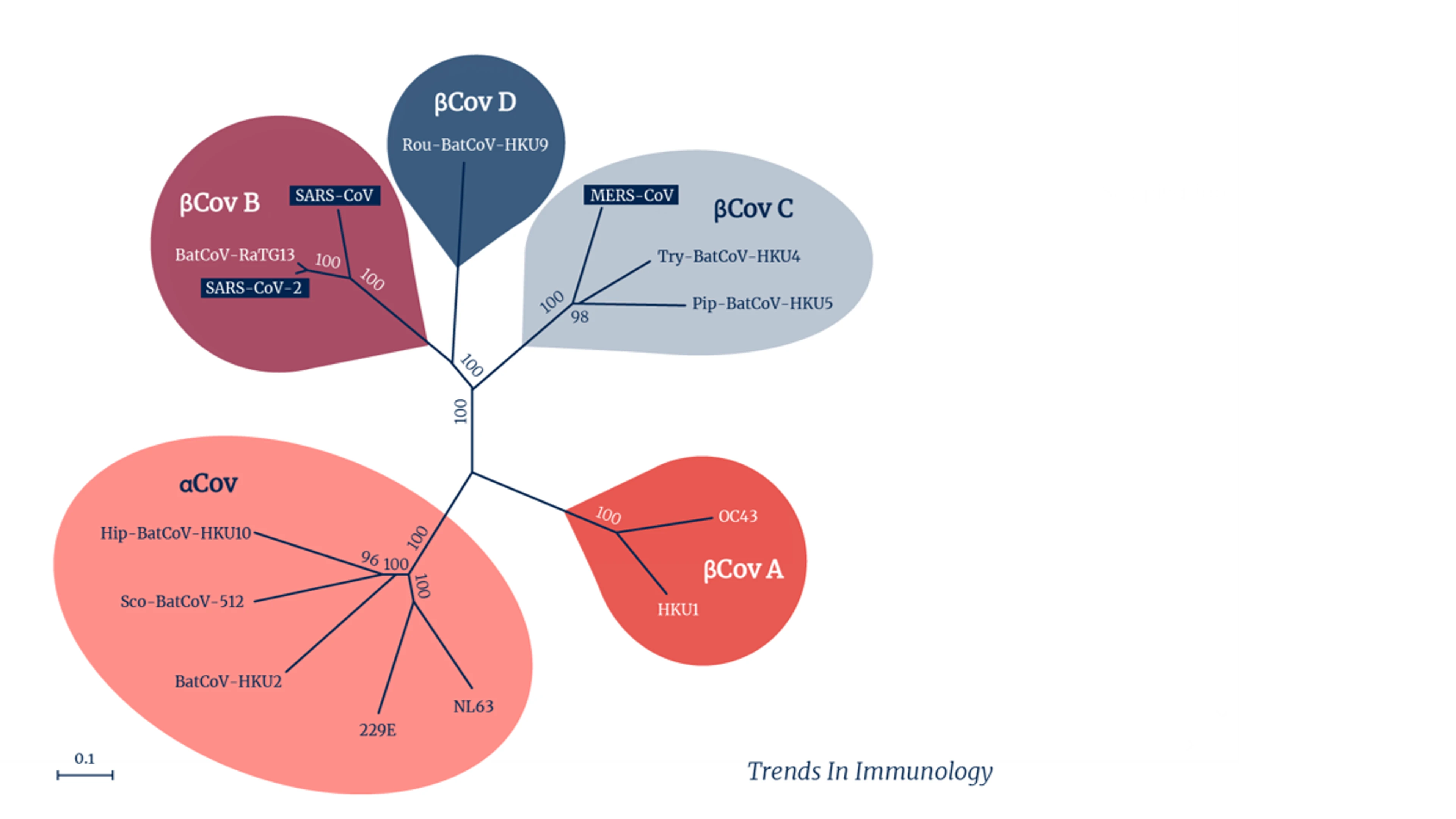
Coronavirus groups and subsets. Credit: Trends in Immunology.
Immunogen design
At the core of the scientific problem is immunogen design: how to move on from vaccines that focus solely on prominent bits of the coronavirus' infamous ‘spike' protein — parts we now know are frustratingly vulnerable to mutation — to find areas that are more stable and hence more likely to ensure a vaccine can protect against more than just one coronavirus and against multiple variants.
"We now know that the spike will evolve and change over time. So, to get beyond that, we need to look for conserved epitopes, (stable parts of the viral antigens that are common across several variants and several coronaviruses)" says Saville. "But usually those conserved epitopes are hidden, so a lot of the work being done at the moment is to try and expose them"
Adds Chris da Costa: "The idea is to have common structural elements of coronaviruses that are conserved across different sub-groups as they evolve over time".
That way, even when the more exposed parts of the coronavirus's spike pick up mutations, a vaccine would still elicit an immune response because it targets parts that remain conserved as the virus mutates.
We want to have several shots on goal, so we've made sure we have a nice mix of technologies and antigen designs.
At this stage, while the science is still in the discovery phase and the best and fastest ways forward have yet to be found, scientists are exploring a whole kaleidoscope of different vaccine technologies. While some teams are working with mRNA-based projects, others are exploring mosaic protein nanoparticle-based vaccine candidates and others are taking a chimeric approach. Japan's NEC, whose Board Chairman Nobuhiro Endo has declared that "it is crucial that we arm ourselves against future invisible enemies"- and its Norwegian subsidiary NEC OncoImmunity AS are pursuing artificial intelligence-powered design of immunogens to find new vaccine antigens that have broad reactivity against betacoronaviruses. "We want to have several shots on goal, so we've made sure we have a nice mix of technologies and antigen designs," says da Costa.
Among those is a project to develop a novel protein nanoparticle vaccine, led by Pamela Bjorkman and colleagues at the California Institute of Technology, or CalTech, which is already showing great promise in preclinical (animal) studies. This potential product, known as a "Mosaic-8" vaccine, uses protein nanoparticles containing a type of protein ‘glue' that attaches to antigenic sections of the spike proteins from eight different coronaviruses in the sarbecovirus sub-group — including the Beta variant of SARS-CoV-2, and seven other coronaviruses that circulate in bats and pangolins.
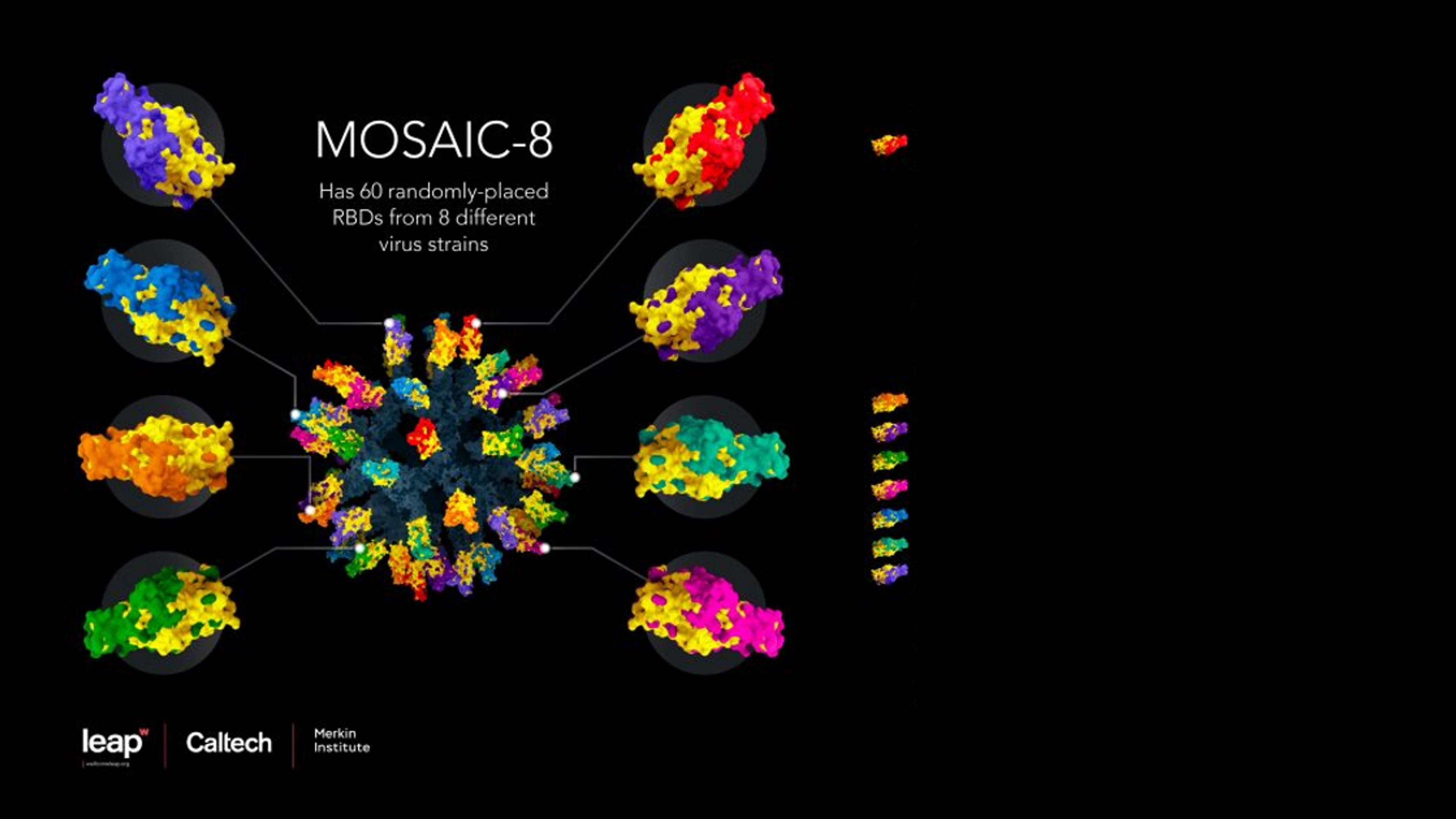
Mosaic-8 nanoparticle vaccine design. Credit: Courtesy of Wellcome Leap, Caltech, and Merkin Institute.
The idea here is that by making a nanoparticle that presents not one but eight different coronavirus pieces - actually sarbecovirus pieces - we train the immune system to make antibodies that are not primarily directed against the immunodominant (and variable) places on those viral fragments. Instead the antibodies are directed against more conserved places," says Professor Pamela Bjorkman, a CalTech professor of biology and biological engineering. "If you make the antibodies against conserved parts, then it shouldn't matter which sarbecovirus virus emerges next, it (the new vaccine) will work against it." In other words, if another SARS or COVID-19 emerged in the coming years, we could already be many steps ahead of it.
In South Korea, CEPI has extended an existing partnership with SK Bioscience to develop a ‘variant-proof' vaccine candidate against sarbecoviruses - the subgroup of viruses that includes both the original SARS as well as SARS-CoV-2. This candidate is a recombinant-protein vaccine based on a two-component self-assembling nanoparticle platform developed at the Institute for Protein Design at the University of Washington. The hope is that this vaccine, which displays receptor-binding domains (the part of the spike protein that enables the virus to bind to and infect human cells) from multiple sarbecoviruses, will prime the immune system to recognise and defend against past, current and future SARS-type viruses and their variants.
If you make the antibodies against conserved parts, then it shouldn't matter which sarbecovirus virus emerges next, it (the new vaccine) will work against it.
Another CEPI-funded potential broadly protective vaccine candidate is a project led by DIOSynVax — a biotech spinout from Britain's University of Cambridge. Theirs is an mRNA vaccine candidate that targets elements of viral structures that are common to all known betacoronaviruses. Because these structures are critical to the virus being able to survive and replicate, the expectation is that they are less likely to change or mutate much when a circulating coronavirus virus spawns future variants or when a new coronavirus emerges. As with other broadly protective vaccine development projects, the idea is that once the body's immune system is presented with these antigens, it will produce neutralising antibodies and killer T-cells against a range of betacoronaviruses, including, hopefully, viruses that have yet to make the jump from animals into people.
What we're striving for is something that can be broadly protective not only against what's circulating now, but also in the future as the virus mutates. (That's something) we have never really been able to achieve with any vaccine.
A consortium led by BioNet, a French-Thai vaccine manufacturer and member of the Developing Country Vaccine Manufacturing Network (DCVMN), is working on a potential vaccine that uses multiple mRNA molecules that encode for several SARS-CoV-2 target proteins, or immunogens, from several different variants. The hope is that this new vaccine could be an effective variant-proof COVID-19 shot, and could also serve as a model for how to make similar mRNA-based vaccines against other betacoronaviruses like MERS-CoV, as well as vaccines against future pathogenic threats that have yet to emerge.
In Canada, the University of Saskatchewan's Vaccine and Infectious Disease Organization (VIDO) is working on a multivalent-type approach which takes different parts of a number of variants and combines them with an adjuvant - a type of vaccine super-charger. "It's definitely a high risk, high reward project," said Dr Trina Racine, Director of Vaccine Development. "What we're striving for is something that can be broadly protective not only against what's circulating now, but also in the future as the virus mutates, so that we don't have to be constantly updating. (That's something) we have never really been able to achieve with any vaccine."
[embed]http://vimeo.com/746856717/c17b10b06c[/embed]
All-in-one vaccines for all
It's still early days in this highly complex work, but some of CEPI's broadly protective coronavirus vaccine partnerships are already seeing exciting results. The latest findings on the CalTech Mosaic 8 coronavirus vaccine candidate (which was developed with technology from the University of Oxford) showed that it provided broad protection in animals both against the viruses included in it, and beyond them against other coronaviruses not specifically targeted. "Mosaic-8 immunization induces neutralizing antibodies against the Beta and Delta variants of SARS-CoV-2, and we found that it also works against the COVID variants that hadn't yet emerged when we started creating the vaccine," says Professor Bjorkman. "And it also works against animal sarbecoviruses that are more distantly related — which bodes well because they represent a potential coronavirus spillover event."
VIDO too has had some promising data. Findings of studies conducted in hamsters showed that the range of antigens included in that vaccine candidate also provided some broad protection against a number of COVID-19 variants.
With these and other promising data in hand, researchers are already looking ahead at how next generation coronavirus vaccines, if successful, could be made at large scale and at an affordable price.
After all, says CEPI's Director of Access and Private Partnerships Emma Wheatley, developing a broadly protective coronavirus vaccine is only part of the work. "Access is at the heart of our decision-making process at CEPI. We have to be sure we're working towards something that will be able to be made, delivered and administered in all countries for all people — rich and poor — around the world. When we're selecting which vaccines to invest in, all of our partners have to commit to take steps to make the shots globally accessible: it's a critical part of our mission."
For the CalTech project, this forward thinking is very much in progress. Initial manufacturing of the Mosaic 8 candidate vaccine is being led by two UK-based firms, Ingenza and CPI, who Bjorkman says have already worked out ways of making the vaccine in an affordable and deliverable way. Previous studies have shown that similar nanoparticles can be freeze dried into a powder form which is inherently far more stable than a liquid vaccine. "The vision is that we could mail it all over the world. Then it can be brought up into a liquid (rehydrated) and injected, so there would be no cold chain problems whatsoever," Bjorkman explains.
Access is at the heart of our decision-making process at CEPI. We have to be sure we're working towards something that will be able to be made, delivered and administered in all countries for all people – rich and poor – around the world.
DioSynVax's next generation vaccine candidate is also being designed with easier and scalable delivery in mind. It could be administered in what's known as a "needle-free injection" — a short high-pressure blast of air that pushes the vaccine into the skin. If it's successful, it could also be scaled up and manufactured as a powder, making it swifter and cheaper to deliver across the globe.
Needle-free technology, Credit Lloyd Mann, University of Cambridge
Given the extreme intricacy of the pan-coronavirus development efforts, it's unlikely the world will see a broadly protective shot for at least a couple of years. The record-breaking speed accomplished in the development of the "one bug, one drug" first generation COVID-19 vaccines is not going to be achievable when the desired end product is so complex and potentially game-changing.
Several of CEPI's partners are looking towards mid-2023 to start Phase 1 human trials in healthy volunteers, though, and the hope is that mid- and final- stage trials could follow swiftly after that if results from initial human tests are promising.
"It's highly complex, but also scientifically feasible, so we are anticipating that it will take more time," says Saville. "The level of technology and innovation we'll need to use in these broadly protective coronavirus vaccine approaches is really taking things up a notch. But if we're successful, we could take the threat of coronavirus pandemics off the table for good."



