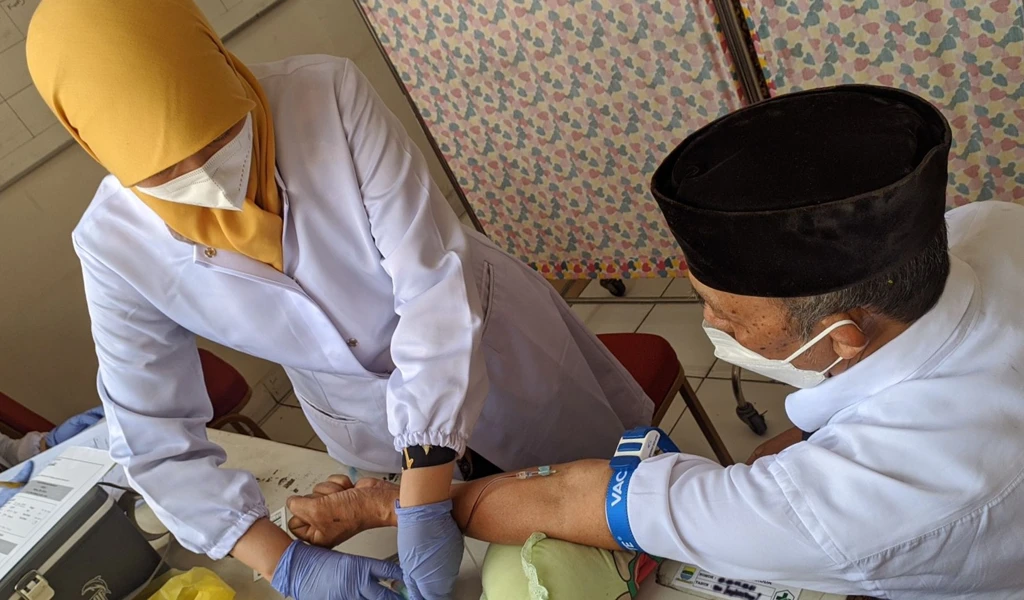
Could chicken eggs help to end the COVID-19 pandemic? That's one of the questions an international consortium of collaborators facilitated by an alliance between the Icahn School of Medicine at Mount Sinai in New York, University of Texas at Austin (UT Austin), and PATH is trying to answer as they develop a new egg-based vaccine to tackle COVID-19, inspired by influenza vaccines in longtime use.
For decades, the most common influenza vaccines have been made by growing flu virus in chicken eggs and inactivating or weakening it so it can't cause disease. In fact, more than a billion safe and effective egg-based flu vaccine doses are made annually worldwide. So, could the egg-based approach be adapted to make a COVID-19 vaccine, and could existing flu vaccine factories be converted to augment the COVID-19 vaccine supply? CEPI is providing financial support of up to $2 million to PATH as the consortium aims to find out.
Renowned U.S. researchers from Icahn Mount Sinai, UT Austin, and PATH are working with manufacturers in Vietnam, Thailand, and Brazil to advance the development of an egg-based COVID-19 vaccine designed to be affordable and manufacturable at scale to increase equitable access. CEPI's funding will support PATH in providing technical assistance to Instituto Butantan in Brazil, the Government Pharmaceutical Organization (GPO) in Thailand, and the Institute of Vaccines and Medical Biologicals (IVAC) in Vietnam — all established flu vaccine manufacturers — who will be suppliers of the vaccine if successful.
Expanding access to COVID-19 vaccines
While huge progress has been made to develop multiple safe and effective COVID-19 vaccines, demand continues to vastly outstrip supply so access to scarce vaccines remains grossly unequal. Adding an egg-based COVID-19 vaccine to the global vaccine arsenal could help improve access in several ways:
Affordability and deliverability. With relatively low production costs and stability at regular refrigerator temperatures (2°C to 8°C) for months, this egg-based vaccine could be a practical platform for reaching resource-limited communities.
Untapped production capacity. Many manufacturers already have the infrastructure to make egg-based vaccines, including in low- and middle-income parts of the world. These facilities could be converted to make large volumes of COVID-19 vaccines during idle periods half the year when they aren't making seasonal flu vaccines, or even more often if necessary. Capacity in Vietnam, Thailand, and Brazil combined could yield hundreds of millions of doses per year.
Greater supply control for countries. As an option that countries could produce themselves or export regionally, an egg-based COVID-19 vaccine could both address the pandemic and provide a reliable backbone for routine immunization. It would give more countries access without having to wait in line behind much wealthier nations—a problem that COVID-19 brought to light. Having homegrown vaccines would reduce dependency on expensive and scarce imported vaccines.
Classic technology meets cutting-edge science
Early clinical trials are already underway in Vietnam, Thailand, and Brazil to evaluate the new egg-based COVID-19 vaccine and the results will inform future development strategies.
The vaccine is based on a novel platform that brings together traditional egg-based technology with new, cutting-edge science—and uses another virus as a building block. Mount Sinai scientists genetically modify a Newcastle disease virus (NDV)—usually used in its live form, in vaccines for poultry—so that its surface presents the SARS-CoV-2 stabilized spike protein (known as HexaPro), developed by UT Austin. SARS-CoV-2 is the coronavirus responsible for causing COVID-19. The spike protein is what trains the immune system to recognize and fight COVID-19 infection. The NDV is grown in eggs, purified, inactivated (or killed), and formulated into an injectable vaccine.
An international collaboration
This one-of-a-kind vaccine candidate would not be possible without international collaboration.
The clinical studies and overall development of the vaccine are being driven by the country manufacturers IVAC, GPO, and Instituto Butantan. Icahn Mount Sinai, UT Austin, and PATH are helping the manufacturers accelerate access to the NDV vaccine and HexaPro technologies. The recombinant NDV technology was developed by Drs. Peter Palese, Adolfo Garcia-Sastre, and Florian Krammer at Icahn Mount Sinai. And, a team at UT Austin led by Drs. Jason McLellan, Ilya Finkelstein, and Jennifer Maynard developed the stabilized spike protein—an earlier version of which forms the basis of several other widely used COVID-19 vaccines. Icahn Mount Sinai provided the starting materials (including the GMP master seed virus), which the manufacturers can readily inject into eggs and scale-up production quickly to potentially provide large quantities of vaccines at low-cost.
Overall, CEPI is supporting PATH's role as connector, facilitator, and technical advisor—linking the technologies and partners and contributing clinical, regulatory, and quality standards expertise in an advisory role across the consortium.
Some of the content in this blog is adapted from an original post on the PATH website.