CEPI to fund three programmes to develop vaccines against the novel coronavirus, nCoV-2019
OSLO, NORWAY. Jan 23, 2020 - CEPI, the Coalition for Epidemic Preparedness Innovations, today announced the initiation of three programmes to develop vaccines against the novel coronavirus, nCoV-2019.
The programmes will leverage rapid response platforms already supported by CEPI as well as a new partnership. The aim is to advance nCoV-2019 vaccine candidates into clinical testing as quickly as possible.
The nCoV-2019 vaccine development efforts will build on existing partnerships with Inovio (Nasdaq: INO) and The University of Queensland (located in Brisbane, Australia). In addition, CEPI today announces a new partnership with Moderna, Inc., (Nasdaq: MRNA) and the U.S. National Institute of Allergy and Infectious Diseases.
All of these are pioneering technologies designed to speed up the development of vaccines against emerging threats such as nCoV-2019.
Given the rapid global spread of the nCoV-2019 virus the world needs to act quickly and in unity totackle this disease. Our intention with this work is to leverage our work on the MERS coronavirus andrapid response platforms to speed up vaccine development. There are no guarantees of success, but we hope this work could provide a significant and importantstep forward in developing a vaccine for this disease. Our aspiration with these technologies is to bringa new pathogen from gene sequence to clinical testing in 16 weeks – which is significantly shorterthan where we are now.
The term "platform technology" broadly refers to systems that use the same basic components as a backbone but can be adapted for use against different pathogens as needed by inserting new genetic or protein sequences.
CEPI has moved with great urgency and in coordination with WHO, who is leading the development of a coordinated international response, to promote the development of new vaccines against the emerging threat of nCoV-2019. The novel coronavirus represents the first new epidemic disease of note to emerge since CEPI's founding at Davos in 2017, with the express intent that it should be ready to respond to epidemics rapidly and effectively, wherever they emerge.
Inovio | DNA vaccine candidate against Middle East Respiratory Syndrome
CEPI announced a partnering agreement, worth up to US$56 million, with Inovio in April 2018, to advance DNA vaccine candidates against MERS and another of its priority diseases, Lassa fever, through to Phase 2.
Under the agreement, funding will support the development up to the end of Phase 2, providing clinical safety, immunological data, and the establishment of investigational stockpiles that will be ready for clinical efficacy trial testing during outbreaks.
The MERS DNA vaccine candidate is being developed using Inovio's DNA Medicines platform to deliver optimised synthetic antigenic genes into cells, where they are translated into protein antigens that activate an individual's immune system to generate robust targeted T cell and antibody responses. Inovio's immunotherapies function exclusively in vivo, and have generated an antigen-specific immune response against targeted diseases in all clinical trials to date.
Inovio is advancing its MERS vaccine candidate into Phase 2, in the Middle East where most MERS viral outbreaks have occurred, with the support of its collaborators: The Wistar Institute, Laval University, the NIH's Rocky Mountain Laboratories, U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), VGXI/GeneOne Life Science and the International Vaccine Institute.
University of Queensland | The molecular clamp platform
CEPI entered a partnering agreement in January 2019, with University of Queensland, for up to US$ 10.6 million to develop a "molecular clamp" vaccine platform, a transformative technology that enables targeted and rapid vaccine production against multiple viral pathogens.
The technology works by synthesising viral surface proteins, which attach to host cells during infection, and "clamping" them into shape, making it easier for the immune system to recognise them as the correct antigen. This process requires the sequence of the viral protein which can then be determined from the viral genome. The synthetic antigen can then be purified and rapidly manufactured into a vaccine.
As part of their partnering agreement with CEPI, the University of Queensland will use their molecular-clamp vaccine platform to produce vaccines against known pathogens, including Middle East Respiratory Syndrome coronavirus (MERS-CoV) and will evaluate the safety and immune response of the Influenza and MERS-CoV candidates in a phase 1 clinical trial in humans.
Moderna | mRNA vaccine platform
Under the terms of the agreement, Moderna will manufacture an mRNA vaccine against 2019-nCoV, which will be funded by CEPI. The Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID), part of NIH, collaborated with Moderna to design the vaccine. NIAID will conduct IND-enabling studies and a Phase 1 clinical study in the U.S.
- ENDS -
About the novel coronavirus
Coronaviruses are a family of viruses that can lead to respiratory illness, including Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). Coronaviruses are transmitted between animals and people and can evolve into strains not previously identified in humans. On January 7, 2020, a novel coronavirus (2019-nCoV) was identified as the cause of pneumonia cases in Wuhan City, Hubei Province of China, and additional cases have been found in a growing number of countries.
About vaccine platform technology
The term "vaccine platform technology" broadly refers to a system that uses the same basic components as a backbone, but can be adapted for use against different pathogens by inserting new sequences.
About "molecular clamp" vaccines
Enveloped viruses, like influenza, have proteins on their surface that fuse to host cells during an infection. Although these surface proteins are antigenic—and therefore elicit an immune response—they are inherently unstable. One approach to vaccine design is to synthesise these proteins on their own such that they elicit an immune response, specifically antibodies, that can kill the virus. Unfortunately, they tend to change shape when expressed on their own, a shape that does not reflect the form of the protein on the virus surface. Consequently, the immune response that is induced with these vaccines does not produce antibodies that efficiently lock on to the virus. The University of Queensland has developed a process that can synthesise these surface proteins while "clamping" them into shape, making it easier for the immune system to induce a response that recognises them on the virus surface.
This synthetic antigen can then be purified and rapidly manufactured into a vaccine, within 16 weeks from pathogen identification.
This vaccine platform technology can be used to develop vaccines against a wide range of enveloped viruses (eg, Influenza, Ebola, MERS, Lassa virus, Measles, Herpes Simplex virus, Rabies).
The Molecular Clamp is patented technology developed by Professor Paul Young, Dr Keith Chappell, and Dr Dan Watterson.
The University of Queensland will be developing this vaccine platform in collaboration with The Commonwealth Scientific and Industrial Research Organisation (CSIRO) and a wider consortium including public sector and private sector partners in Australia, USA, and Asia.
About CEPI
CEPI is an innovative partnership between public, private, philanthropic, and civil organisations, launched at Davos in 2017, to develop vaccines to stop future epidemics. CEPI has reached over US$750 million of its $1 billion funding target. CEPI's priority diseases include Ebola virus, Lassa virus, Middle East Respiratory Syndrome coronavirus, Nipah virus, Rift Valley Fever and Chikungunya virus. CEPI also invests in platform technologies that can be used for rapid vaccine and immunoprophylactic development against unknown pathogens (ie, Disease X). To date, CEPI has committed to investing over $456 million in vaccine and platform development. Learn more at www.cepi.net. Follow us at @CEPIvaccines.
About Inovio Pharmaceuticals, Inc.
Inovio is taking immunotherapy to the next level in the fight against cancer and infectious diseases. We are the only immunotherapy company that has reported generating CD8+ T cells in vivo in high quantity that are fully functional and whose killing capacity correlates with relevant clinical outcomes with a favorable safety profile. With an expanding portfolio of immune therapies, the company is advancing a growing clinical stage product pipeline, including candidates in Phase 3 and Phase 2. Partners and collaborators include MedImmune, Regeneron, Genentech, The Wistar Institute, University of Pennsylvania, the Parker Institute for Cancer Immunotherapy, DARPA, GeneOne Life Science, Plumbline Life Sciences, ApolloBio Corporation, Drexel University, NIH, HIV Vaccines Trial Network, National Cancer Institute, U.S. Military HIV Research Program, and Laval University. For more information, visit www.inovio.com.
About University of Queensland UQ rates in the global top 50 as measured by the Permance Ranking of Scientific Papers for World Universities and was recently rated 7th in Biotechnology world in the Shanghai Global Rankings of 2017. Professor Paul Young, Dr Keith Chappell, and Dr Dan Watterson have extensive expertise in molecular virology, viral pathogenesis and vaccine research.
About Moderna
Moderna is advancing messenger RNA (mRNA) science to create a new class of transformative medicines for patients. mRNA medicines are designed to direct the body's cells to produce intracellular, membrane or secreted proteins that have a therapeutic or preventive benefit with the potential to address a broad spectrum of diseases. Moderna's platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, providing the Company the capability to pursue in parallel a robust pipeline of new development candidates. Moderna is developing therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and cardiovascular diseases, and autoimmune and inflammatory diseases, independently and with strategic collaborators.
Headquartered in Cambridge, Mass., Moderna currently has strategic alliances for development programs with AstraZeneca, Plc. (NASDAQ: AZN) and Merck, Inc. (NASDAQ: MRK), as well as the Defense Advanced Research Projects Agency (NASDAQ: DARPA), an agency of the U.S. Department of Defense and the Biomedical Advanced Research and Development Authority (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS). Moderna has been named a top biopharmaceutical employer by Science for the past five years. To learn more, visit www.modernatx.com.
For media enquiries, please contact:
Rachel Grant, Director of Communications and Advocacy, CEPI
Tel: +44(0)7891249190
Email: [email protected]
Mario Christodoulou, Communications and Advocacy Manager, CEPI
Tel: +44 (0) 7979300222
Email: [email protected]
Jodie Rogers, Communications Officer, CEPI
Tel: +44(0)79 793 57 459
Email: [email protected]
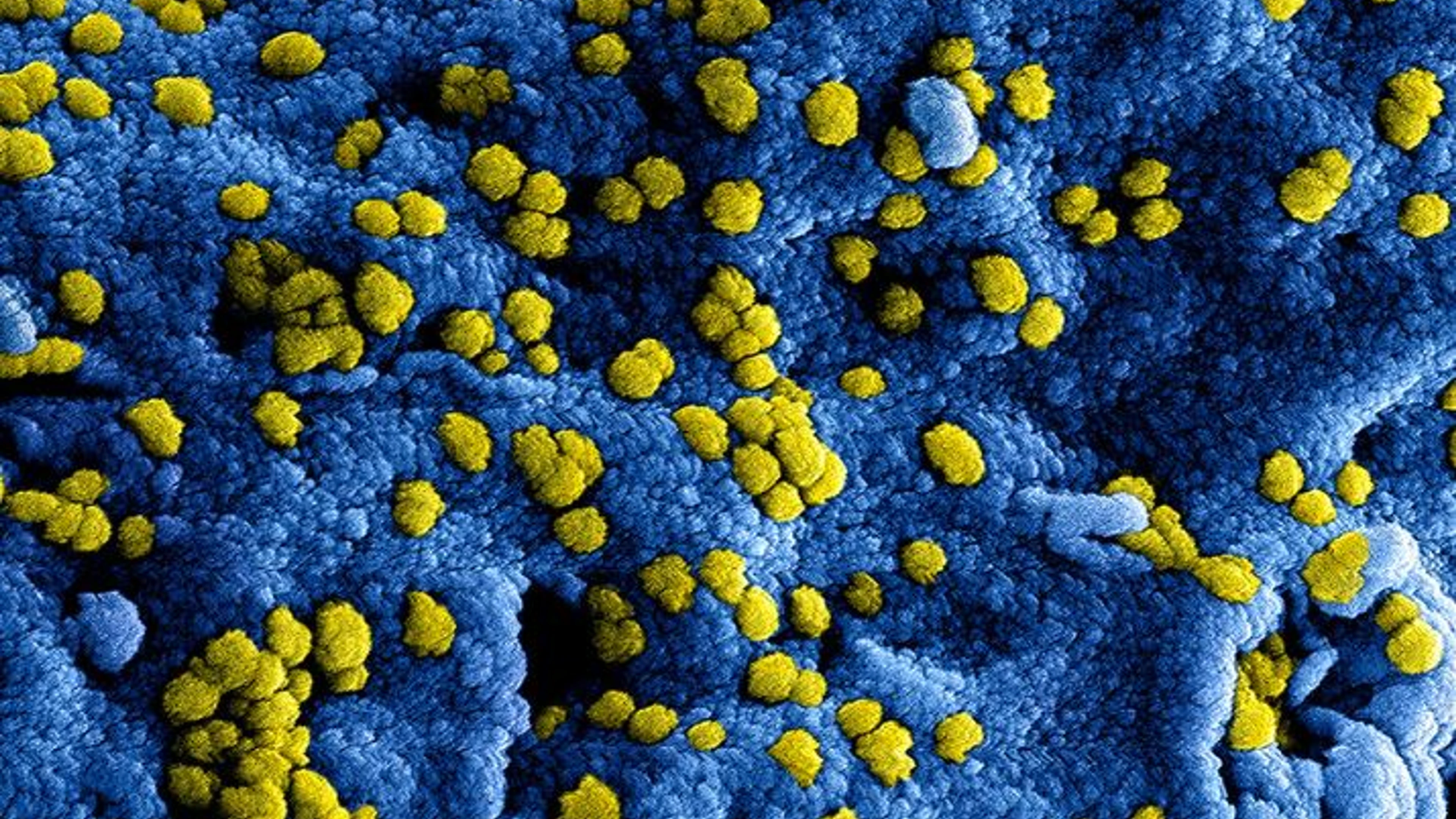
Image caption and credit: Colorized scanning electron micrograph of Middle East Respiratory Syndrome virus particles attached to the surface of an infected VERO E6 cell. Image captured and color-enhanced at the NIAID Integrated Research Facility in Ft. Detrick, Maryland. Credit NIAID