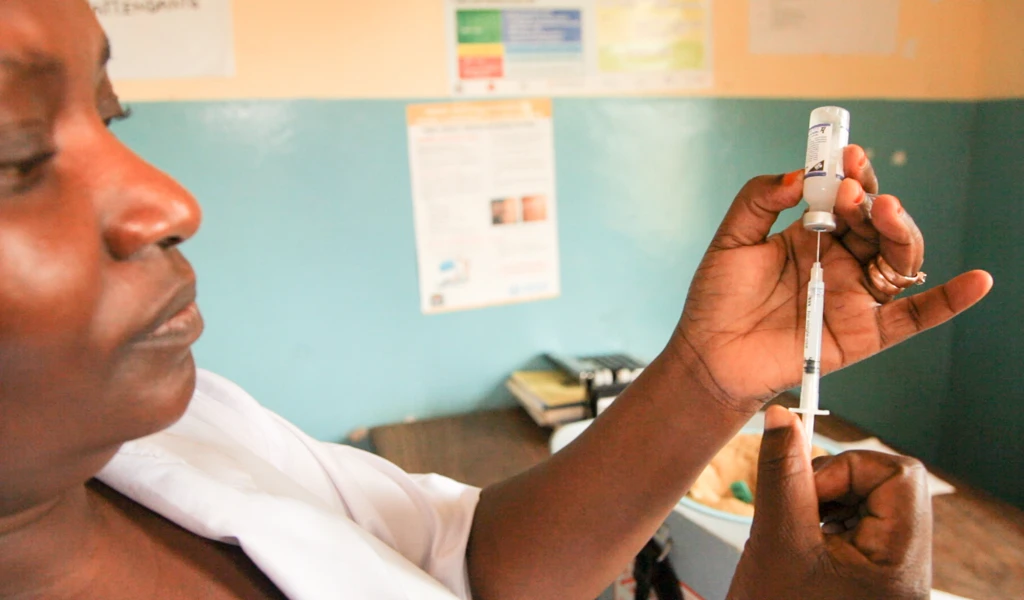
CEPI partners with AstraZeneca to manufacture 300 million globally accessible doses of COVID-19 vaccine
June 4 2020, Oslo, Norway—CEPI, the Coalition for Epidemic Preparedness Innovations, has today announced a partnership with AstraZeneca (LSE/STO/NYSE:AZN) which will support the manufacture of 300 million doses of the AZD1222 vaccine candidate to be ringfenced for the COVID-19 Vaccine Global Access Facility* if the vaccine is proven to be safe and effective.
CEPI will invest a total of up to $383m in this partnership. This funding will support the technical transfer of vaccine production technology to manufacturing sites predominantly in Europe, thereby creating additional manufacturing capacity for this vaccine. It will also purchase manufacturing materials and reserve manufacturing slots, securing a total 300 million doses of vaccine for the COVID-19 Vaccine Global Access Facility.
CEPI and AstraZeneca are united in their commitment to equitable access to the vaccine. AstraZeneca and CEPI's ACT Accelerator partner Gavi have entered into a Memorandum of Understanding which envisages procuring the vaccine produced under the CEPI agreement for distribution by the COVID-19 Vaccine Global Access Facility. AstraZeneca will supply the vaccine to the COVID-19 Vaccine Global Access Facility on a no profit basis during the pandemic.
AZD1222 has now progressed into late-stage Phase II/III clinical trials in more than 10,000 people from across the UK. If the vaccine is proven to be safe and effective, the first doses to be produced under this agreement are anticipated to be available in early 2021. Vaccines will be released on a rolling basis as production is completed, and the full quota of 300 million doses is expected to be available by July 2021.
This agreement builds upon CEPI's initial seed funding for this vaccine candidate which supported the University of Oxford to manufacture clinical trial materials, in addition to funding preclinical studies of AZD1222 at Australia's Commonwealth Scientific and Industrial Research Organization.
CEPI supported the early development of this vaccine candidate, contributing to the promising stage of development it has reached today. If it is proven to be safe and effective the vaccine could be amongst the first to be licensed, and CEPI's partnership with AstraZeneca will make hundreds of millions of doses of the vaccine accessible to those at the highest risk.
AstraZeneca and our other industry partners have a critical role to play in rapidly developing safe and effective vaccines and manufacturing the billions of doses needed to put a permanent end to the COVID-19 pandemic. AstraZeneca is admirably committed to equitable global access for this vaccine, and this partnership demonstrates how the COVID-19 Vaccine Global Access Facility will bring the private, public and third sectors together to make COVID-19 vaccines available to those who need them most, for the benefit of all.
We are working tirelessly to honour our commitment to ensure broad and equitable access to Oxford's vaccine across the globe and at no profit. Today marks an important step in helping us supply hundreds of millions of people around the world, including to those in countries with the lowest means. I am deeply grateful for everyone's commitment to this cause and for their work in bringing this together in such a short time.
CEPI's COVID-19 portfolio
To date, CEPI has provided support and funding to develop COVID-19 vaccine candidates to Curevac, Inc., Inovio Pharmaceuticals, Inc., Moderna, Inc., Novavax, Inc. , The University of Queensland, The University of Hong Kong, The University of Oxford, a consortium led by Institut Pasteur, and Clover Biopharmaceuticals.
*The COVID-19 Vaccine Global Access Facility is an instrument of the Vaccines pillar of the ACT Accelerator within which CEPI works in partnership with Gavi and the World Health Organisation.
-ENDS-
About CEPI
CEPI is an innovative partnership between public, private, philanthropic, and civil organisations, launched at Davos in 2017, to develop vaccines to stop future epidemics. CEPI has moved with great urgency and in coordination with WHO in response to the emergence of COVID-19. CEPI has initiated 9 partnerships to develop vaccines against the novel coronavirus. The programmes will leverage rapid response platforms already supported by CEPI as well as new partnerships. The aim is to advance COVID-19 vaccine candidates into clinical testing as quickly as possible.
Before the emergence of COVID-19 CEPI's priority diseases included Ebola virus, Lassa virus, Middle East Respiratory Syndrome coronavirus, Nipah virus, Rift Valley Fever and Chikungunya virus. CEPI also invested in platform technologies that can be used for rapid vaccine and immunoprophylactic development against unknown pathogens (Disease X).
Follow our news page for the latest updates.
Media contacts
CEPI:
Email: [email protected]
Phone: +44 7387 055214
Image caption: Bottles on the bottling line of the pharmaceutical plant. Credit: Cergios/ ShutterStock