CEPI funds consortium led by CPI to advance Caltech's new all-in-one coronavirus vaccine

Up to $30m funding will be provided to advance an innovative vaccine technology that could protect against current and future SARS-CoV-2 variants and other SARS-like Betacoronaviruses.
New peer-reviewed research, out today in Science, demonstrates the vaccine platform provides protection against a spectrum of SARS-like Betacoronaviruses in preclinical models.
This is the eleventh award to be made by CEPI as part of its broadly protective coronavirus vaccine programme, making the coalition a global leader in all-in-one coronavirus vaccine research.
Jul 5, 2022 — The Coalition for Epidemic Preparedness Innovations (CEPI) has partnered with a consortium of research and technological institutions to develop a novel vaccine to provide protection against COVID-19 caused by current and future SARS-CoV-2 variants, as well as to protect against other SARS-like Betacoronaviruses*.
The project will be led in manufacturing efforts by UK-based deep tech innovation organisation CPI to advance the novel vaccine developed at Caltech and The University of Oxford, and manufactured using microbes engineered by industrial biotechnology leader, Ingenza Ltd.
CEPI will provide up to US $30 million to support the development of the vaccine through Phase I trials, including preclinical work, with the aim to establish first-in-human clinical proof of concept for the vaccine. The funding will also support the design of the vaccine and regulatory activities.
Recognising the risk of future coronavirus threats, CEPI has established itself as the global leader in funding all-in-one coronavirus vaccine research. This is the eleventh award to be made as part of CEPI's US $200 million programme supporting projects that could provide broad protection against SARS-CoV-2 variants and other Betacoronaviruses. CEPI also today announced another broadly protective coronavirus vaccine partnership with Codiak Biosciences. The programme is a central part of CEPI's innovative US $3.5 billion pandemic preparedness plan that seeks to reduce, or even eliminate, the threat of future pandemics.
An innovative coronavirus-fighting technology
Many COVID-19 vaccine candidates work by presenting fragments of the SARS-CoV-2 spike protein (known as the receptor-binding domain, or RBD) to the body to generate an immune response. However, newly arising SARS-CoV-2 variants, such as various forms of Omicron, are increasingly able to evade the original vaccine's immune response. Changeable regions of their RBDs have been mutated in ways that allow them to escape from the original immune response.
By contrast, this new vaccine aims to create immunity to known and future strains simultaneously is designed to focus the immune response on parts of RBD shared by all viruses in the SARS-like Betacoronavirus family, including future variants. It does this by presenting spike protein fragment RBDs from SARS-CoV-2 together with RBDs from seven other different types of coronaviruses on protein nanoparticles termed "mosaic-8" nanoparticles.
These coronavirus RBDs are attached to a nanoparticle by protein tags, enabling the structure to display fragments from multiple viruses on a single nanoparticle. The nanoparticle contains a protein "glue" on its surface that attaches to engineered coronavirus fragments like ‘velcro,' an innovative approach to permanently link proteins to each other.
Supporting this approach, new research published today in Science, demonstrates that this mosaic-8 nanoparticle vaccine technology elicits protective immune responses in preclinical models against SARS-like Betacoronaviruses with components displayed on the mosaic nanoparticle as well as coronaviruses from which no components were displayed. This included SARS-like Betacoronaviruses seen in animals with the potential to spillover to humans. These findings suggest that the technology may also provide protection against future novel SARS-CoV-2 variants and as-yet-undiscovered coronaviruses that could transfer into the human population in the future.
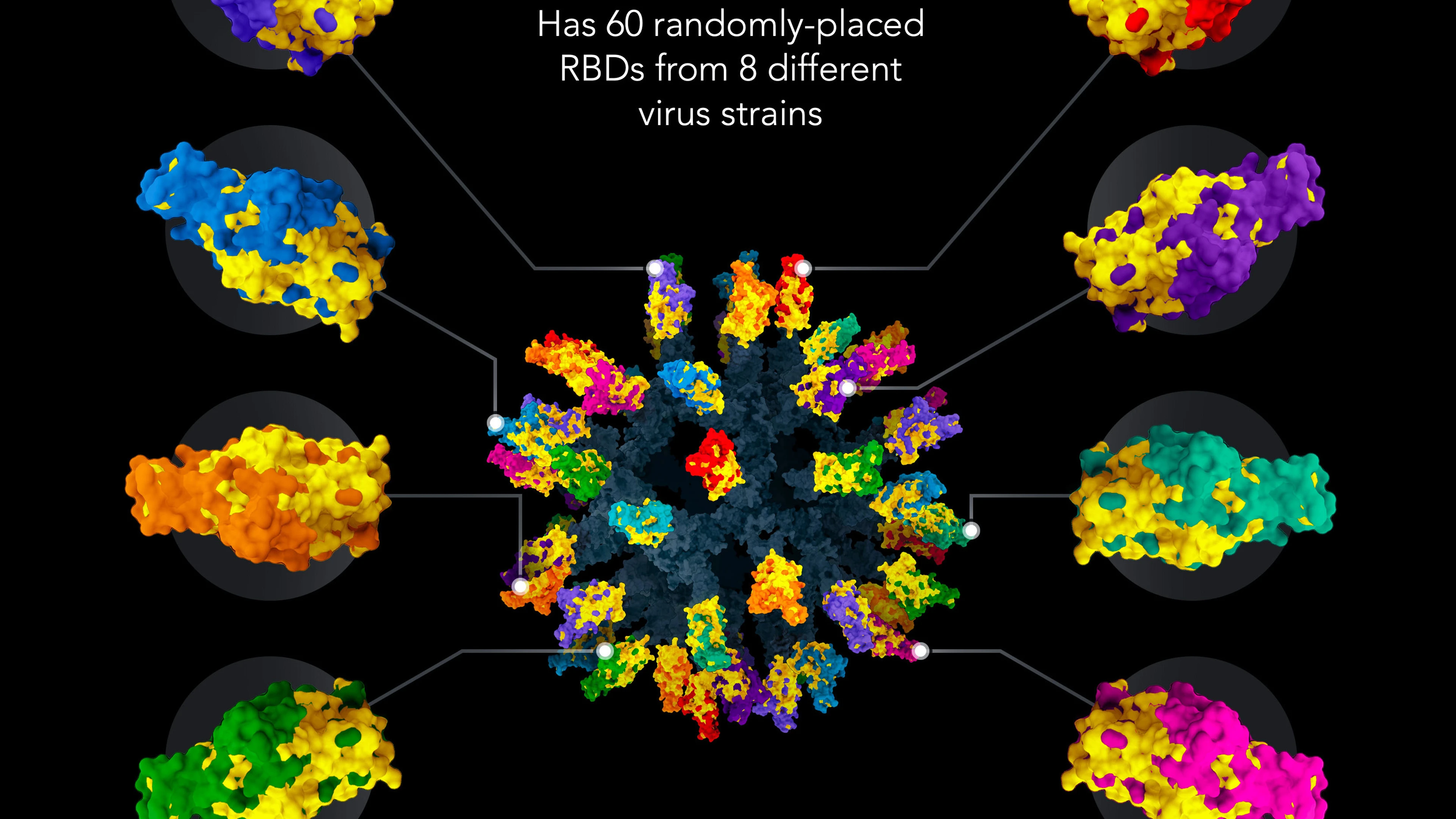
Image courtesy of Wellcome Leap, Caltech, and Merkin Institute. ©2022. All rights reserved.
Dr Richard Hatchett, CEO of CEPI, said: "There have already been three serious coronavirus epidemics or pandemics in the twenty-first century — and COVID-19 continues to have a devastating impact on the world's health, society, and economy.
"The creation of vaccines that could provide broad protection against emerging COVID-19 variants and future coronavirus threats would not only help mitigate the damaging effects of another COVID-19-like pandemic, it could also help reduce the time taken and funding spent continually updating vaccine formulations.
"That's why we are delighted to today partner with this CPI-led innovation consortium to build on Wellcome Leap's initial investment in Caltech's technology and further advance this pioneering mosaic nanoparticle vaccine approach that, if successful, could work towards consigning the threat posed by coronaviruses to the history books."
Kris Wadrop, CPI General Manager and the Project Lead, said: "CPI has been working together with these partners since mid-2020 to develop this type of vaccine to ensure it can be manufactured in the quantities and price range required to have a real impact everywhere. Confirmation of this funding is a testament to the team's diligence and tenacity to highlight the potential offered by an RBD-nanoparticle vaccine."
"The strength assembled in this world-class team is providing excellence in research and science with pragmatism in production technology and market knowledge. We are really excited to be working with our partners and leading this consortium from the Tees Valley, in Northeast England."
Dr. Pamela Bjorkman, the Project Lead and David Baltimore Professor of Biology and Biological Engineering and Merkin Institute Professor at Caltech, said: "I am grateful for support from Caltech's Merkin Institute for Translational Research that allowed my lab to start the mosaic-8 project shortly after the COVID-19 pandemic began, to Wellcome Leap for their support of preclinical studies in non-human primates, and to the Bill and Melinda Gates Foundation for on-going support. We are now thrilled to be working with CEPI and our colleagues at Oxford, Ingenza, and CPI to move this vaccine candidate into the clinic. We are very encouraged by preclinical results assessing the mosaic RBD nanoparticle approach, which has demonstrated advantages over current vaccines and traditional single RBD nanoparticle vaccine candidates. Our consortium has already combined its diverse skill sets to implement new ideas and scientific advances, and we look forward to continuing to work with our wonderful collaborators."
Prof. Alain Townsend, Professor of Molecular Immunology at the MRC Human Immunology Unit, University of Oxford, said: "The evolution of this consortium is an example of collaborative science at its best. We had been deeply impressed by the power of the protein "glue" for sticking proteins together, and using this technology developed at University of Oxford, my lab made a prototype nanoparticle SARS-CoV-2 vaccine that induced highly potent responses in preclinical studies. Through connections made by Ian Wilkinson (Absolute Antibody), we joined with colleagues at Ingenza and CPI who succeeded in producing a fully functional vaccine produced in microbes, reducing the cost of production. We have been collaborating with Prof. Pamela Bjorkman and the Caltech team, who had independently developed the brilliant concept of the mosaic version of the vaccine and are excited to continue working with this world-class consortium."
Dr. Jack Tan, Senior Postdoctoral Scientist at the MRC Weatherall Institute of Molecular Medicine, University of Oxford, added: "We are excited to be part of the consortium to support the pre-clinical development and move this vaccine candidate into the clinic with the expert guidance of Dr. Parvinder Aley and Prof. Sir Andrew Pollard of the Oxford Vaccine Group. We are delighted to be working with CEPI to further this nanoparticle technology with the goal of producing efficacious, low-cost, infrastructure-independent vaccine that will be accessible to low- and middle-income countries."
Ian Fotheringham, the Managing Director of Ingenza, said: "It has been a pleasure to work within this phenomenal consortium. This program is a validation of Ingenza's consistent and ongoing investment in efficient and sustainable biomanufacturing technologies that will accelerate innovative biologics and vaccine candidates to worldwide deployment. Our commitment to enhancing human health and a sustainable and equitable global environment fundamentally underpins our engagement with these consortium partners to deliver this next-generation vaccine."
Initial development and validation of the prototype mosaic-8 vaccine approach was supported by Wellcome Leap and Caltech's Merkin Institute for Translational Research. Wellcome Leap provided the pivotal support needed to achieve the current results; and, importantly, to maintain the scientific momentum catalysed by Merkin's earlier funding. Acceleration of the preclinical study allowed it to be completed in time to secure funding from CEPI's broadly protective Betacoronavirus vaccine call for the Phase I clinical trials announced today. Taken together, these efforts have the potential to condense the path to full-scale production by three to four years.
Regina E. Dugan, CEO of Wellcome Leap, said: "This early transition success demonstrates the value of global partnerships working collaboratively and with the urgency needed to address future pandemic risks."
CEPI is committed to the principle of equitable access to the vaccines it funds. Under the terms of the agreement, CPI and its consortium partners have committed to achieving equitable access to the outputs of this vaccine project, by way of pricing, volume commitments and potential deployment of the technology to low- and middle-income country manufacturers as needed, all of these in line with CEPI's Equitable Access Policy.
*Betacoronaviruses are types of coronavirus that cause Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), which have been responsible for major epidemics in Asia and the Middle East in recent years, and also SARS-CoV-2, the virus responsible for the ongoing COVID-19 pandemic.
—ENDS—
Notes to Editors
Additional information on today's announcement and the publication in Science can be found in press release of partners Caltech and Wellcome Leap.
Further details on the Mosaic-8 vaccine technology and new partnership
The innovative protein "glue" used as part of the vaccine to attach coronavirus fragments to the nanoparticle like ‘velcro' was developed from basic science studies of the pathogenic bacterium Streptococcus pyogenes by Prof. Mark Howarth at University of Oxford. The technique can be applied beyond coronavirus vaccines, for example in vaccines for HIV and influenza.
Manufacturing of the mosaic-8 vaccine candidate initially required costly and inflexible mammalian systems for the production of coronavirus fragments and the endotoxin-producing bacterium Escherichia coli for the production of the nanoparticle carrier. Ingenza will adapt its Pichia pastoris and Bacillus subtilis manufacturing platforms to prepare each component of this novel vaccine using highly efficient, low-cost, scalable, and endotoxin-free biomanufacturing systems.
By combining Caltech's mosaic approach together with Ingenza's microbial production advances and Oxford's clinical trials expertise and nanoparticle platform, CPI will develop and scale-up this new multi-coronavirus vaccine.
The mosaic-8 vaccine technology has the potential to reduce the significant costs and supply chain complications associated with the production of most vaccines. For example, RNA-based SARS-CoV-2 vaccines must be kept cold, posing challenges for energy, transport, and storage. This new vaccine, however, eliminates the need for cold storage and specialist skills currently concentrated in few nations, therefore helping to reduce inequities through greater global vaccine accessibility.
CEPI's $200m all-in-one coronavirus vaccine programme
Including today's announcement, CEPI has to date announced funding for eleven programmes to advance the development of vaccines that could provide broad protection against SARS-CoV-2 variants and other betacoronaviruses:
MigVax Ltd— funding of US$4.3m to MigVax Ltd to support the initial development of a new orally administered subunit vaccine tablet that could offer broad protection against SARS-CoV-2 variants
University of Saskatchewan's Vaccine and Infectious Disease Organization (VIDO)— funding of US$5m to support the initial development of a new vaccine based on VIDO's novel protein subunit technology that could offer broad protection against SARS-CoV-2 variant
Affinivax— funding of up to $4.5m to support the initial development of a vaccine candidate based on Affinivax's MAPS platform that could offer broad protection against SARS-CoV-2 variants
SK bioscience— funding of up to US$50m to support the development of a vaccine candidate based on SK's nanoparticle vaccine platform to elicit immune responses that could protect against variants of both SARS-CoV, SARS-CoV-2, and others
Translational Health Science and Technology Institute (THSTI) and Panacea Biotec— funding of up to US12.5m to support the development of multi-epitope, nanoparticle-based vaccine candidates that could provide broad protection against SARS-CoV-2 variants and other Betacoronaviruses
BioNet— funding of up to US$16.9m to advance the development of a novel mRNA-based vaccine that could offer broad protection against SARS-CoV-2 variants
DIOSynVax— funding of up to US$42m to support the development of a broadly protective Betacoronavirus vaccine using mRNA platform technology
NEC Corporation — funding of up to US$4.8m to support the initial development of an AI-designed vaccine based on mRNA technology that protects against a broad range of betacoronaviruses.
Bharat Biotech/ University of Sydney/ ExcellGene — funding of up to US$19.3m to support the development of an adjuvanted subunit vaccine designed to provide broad protection against SARS-CoV-2 variants.
Codiak Biosciences — funding of up to US$2.5m to advance their proprietary exoVACC TM platform from its pan Betacoronavirus program through preclinical studies.
CPI-led consortium — funding of up to US$30m to advance the development of the mosaic vaccine coronavirus technology up to Phase I trials, including preclinical work, that could protect against SARS-CoV-2 variants and other SARS-like betacoronaviruses.
Separate to today's announcement, CEPI is working with Wellcome Leap to develop and demonstrate the technology needed to build a global network of ‘living' biofoundries that would increase the number, diversity, affordability, and availability of RNA-based biologics and better prepare for future pandemics.
About CEPI
CEPI is an innovative partnership between public, private, philanthropic, and civil organisations, launched at Davos in 2017, to develop vaccines against future epidemics. Prior to COVID-19, CEPI's work focused on developing vaccines against Ebola virus, Lassa virus, Middle East Respiratory Syndrome coronavirus, Nipah virus, Rift Valley Fever virus and Chikungunya virus — it has over 20 vaccine candidates against these pathogens in development. CEPI has also invested in new platform technologies for rapid vaccine development against unknown pathogens (Disease X).
During the current pandemic, CEPI initiated multiple programmes to develop vaccines against SARS-CoV-2 and its variants with a focus on speed, scale and access. These programmes leverage the rapid response platforms developed by CEPI's partners prior to the emergence of COVID-19 as well as new collaborations. The aim is to advance clinical development of a diverse portfolio of safe and effective COVID-19 candidates and to enable fair allocation to these vaccines worldwide through COVAX.
CEPI's 5-year plan lays out a $3.5 billion roadmap to compress vaccine development timelines to 100 days, develop a universal vaccine against COVID-19 and other Betacoronaviruses, and create a "library" of vaccine candidates for use against known and unknown pathogens. The plan is available at endpandemics.cepi.net.
Follow our news page for the latest updates. Follow us on Twitter @CEPIvaccines, @DrRHatchett, and LinkedIn.
About CPI
CPI takes great ideas and inventions and makes them a reality. Born in the North East of England in 2004, CPI is an independent deep tech innovation organisation and a founding member of the High Value Manufacturing Catapult.
CPI's team of intelligent people use advances in science and technology to solve the biggest global challenges in healthcare and sustainability. Through incredible people and innovation infrastructure, CPI collaborates with partners in industry, academia, government, and the investment community to accelerate the development and commercialisation of innovative products.
CPI's work ranges from health technologies, advanced drug delivery systems, and medicines manufacturing innovations for multiple modalities including small molecules, biologics, and nucleic acids; to developing sustainable materials for energy storage and packaging, as well as novel food, feed, and nutraceuticals, that are all underpinned by digital technology. CPI turns the entrepreneurial spirit and radical thinking of its people and partners into incredible impact that makes our world a better place.
Let's innovate together: uk-cpi.com. Connect with us: LinkedIn Twitter Instagram Facebook
Media Contacts
CEPI
Email: [email protected] | Phone: +44 7387 055214
CPI
Tori Blakeman
Email: [email protected] | Phone: 07800 969343
Caltech
Lori Dajose
Email: [email protected] | Phone: 626-658-0109
University of Oxford
Email: [email protected] | Phone: +44 01865 280528
Ingenza
Sarah Scott
Email: [email protected] | Phone: +44 (0)131 651 9681
Wellcome Leap
Email: [email protected]


