CEPI funds Aga Khan University-led consortium to conduct mix-and-match trial of COVID-19 vaccines in Pakistan
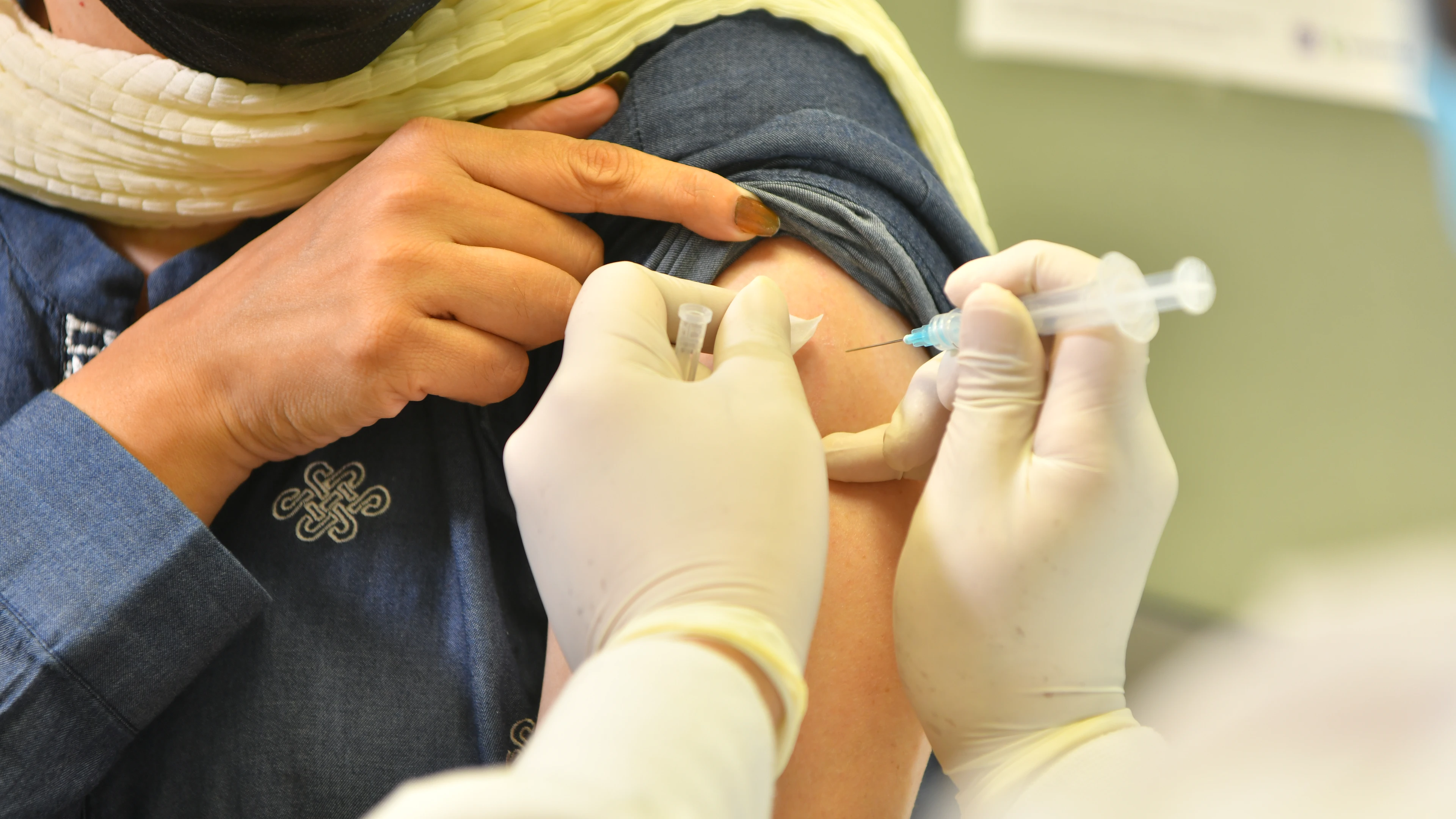
Photo credit: Aga Khan University
23 November 2021; Oslo, Norway and Karachi, Pakistan: The Coalition for Epidemic Preparedness Innovations (CEPI) and the Aga Khan University (AKU) today announced a new collaboration to conduct a clinical trial of heterologous — or ‘mix-and-match' - combinations of COVID-19 vaccines in Pakistan. CEPI will provide up to US$11.7 million of funding for the project to an international consortium led by AKU, comprising the National Institute of Health in Pakistan (NIH), University of Oxford, the International Vaccine Institute (IVI) and Harvard Medical School.
The clinical trial will assess the safety and immunogenicity of mix-and-match combinations of three vaccines that are currently being deployed in Pakistan, developed by AstraZeneca, Sinopharm and CanSinoBIO. Data on mix-and-match combinations of these vaccines, which are commonly used in low- and middle-income countries (LMICs), are urgently needed to contribute to the design of more flexible vaccination strategies and mitigate against shortages of vaccine at times of uncertain or fluctuating supplies. The trial will also generate valuable data on the immune response after vaccination to the different variants circulating in Pakistan. All of the data will be made available open source to inform policy makers and regulatory authorities' recommendations on the use of COVID-19 vaccines.
The Phase 2 clinical trial will be conducted at established trial sites in the cities of Karachi, Lahore and Islamabad, Pakistan. Over 1,600 participants aged 18 years and over will be enrolled into the trial and randomized to receive either a homologous vaccine regimen (two doses of the same vaccine) or a mix-and-match combination of vaccines. All of the possible combinations of the three vaccines will be evaluated in the study. Trial participants will be followed up for two years to gather important data about the durability of immune responses and the impact of possible booster doses. The first interim results are expected to be available in the first quarter of 2022.
While multiple safe and effective COVID-19 vaccines are now in use around the world, additional R&D will help deliver the maximum public health benefit from every dose. The data generated by this clinical trial will contribute to our mission to enable equitable access by supporting the design of vaccination strategies which optimize the use of vaccines in LMICs. We are delighted to begin working with Dr. Qamar of the Aga Khan University, and the National Institute of Health in CEPI's first partnership in Pakistan, in conjunction with our established partners at IVI and the University of Oxford.
Data generated from trials on mix-and-match COVID-19 vaccine combinations is important in informing effective covid vaccination policy in LMICs.
In the light of evolving requirements for COVID-19 vaccination by various countries, this study will bridge gaps in data and provide guidance about combinations of vaccines that are effective and safe.
Expanding access to COVID-19 vaccines by filling R&D gaps
This is the third programme to be funded in response to a CEPI Call for Proposals launched in January 2021 which aims to address current gaps in our clinical knowledge of vaccine performance both now and in the long term, in order to expand access to COVID-19 vaccines as part of the global vaccination rollout. Examples of such gaps include assessment of the safety and effectiveness of COVID-19 vaccines in pregnant women, infants and children, and immunocompromised populations, as well as studies on booster doses, length of vaccine efficacy, ‘mix and match' strategies, and dosing intervals. In response to this Call for Proposals, CEPI is also funding a study of COVID-19 vaccines in immunosuppressed and transplant patients and a project to expand access to BBIBP-CorV in Africa. In addition, CEPI has previously announced funding to support a mix-and-match study led by the University of Oxford
This funding forms part of CEPI's next 5-year plan, published in March 2021, which aims to reduce or even eliminate the future risk of pandemics and epidemics. As part of this plan CEPI is working to strengthen our defences against COVID-19 and reduce the risk of future coronavirus pandemics, by optimizing our current vaccines, addressing variants of concern, developing next-generation COVID-19 vaccines, and initiating the development of broadly protective or universal coronavirus vaccines.
-ENDS-
About CEPI
CEPI is an innovative partnership between public, private, philanthropic, and civil organisations, launched at Davos in 2017, to develop vaccines against future epidemics. Prior to COVID-19 CEPI's work focused on developing vaccines against Ebola virus, Lassa virus, Middle East Respiratory Syndrome coronavirus, Nipah virus, Rift Valley Fever virus and Chikungunya virus — it has over 20 vaccine candidates against these pathogens in development. CEPI has also invested in new platform technologies for rapid vaccine development against unknown pathogens (Disease X).
During the current pandemic, CEPI initiated multiple programmes to develop vaccines against SARS-CoV-2 and its variants with a focus on speed, scale and access. These programmes leverage the rapid response platforms developed by CEPI's partners prior to the emergence of COVID-19 as well as new collaborations. The aim is to advance clinical development of a diverse portfolio of safe and effective COVID-19 candidates and to enable fair allocation to these vaccines worldwide through COVAX.
CEPI's 5-year plan lays out a $3.5 billion roadmap to compress vaccine development timelines to 100 days, develop a universal vaccine against COVID-19 and other Betacoronaviruses, and create a "library" of vaccine candidates for use against known and unknown pathogens. The plan is available at https://endpandemics.cepi.net.
Follow our news page for the latest updates. Follow us via @CEPIvaccines, @DrRHatchett, and LinkedIn.
About the Aga Khan University
The Aga Khan University is a pioneering institution of higher education whose mission is to improve the quality of life in the developing world and beyond, through world-class teaching, research and healthcare delivery. AKU educates students for local and global leadership from campuses and teaching hospitals in six countries, primarily in Asia and Africa. It generates new knowledge to solve problems that affect millions of people, especially the most vulnerable. The University is a private, not-for-profit institution and an agency of the Aga Khan Development Network. www.aku.edu
Media Contacts
CEPI
Email: [email protected]
Phone: +44 7387 055214
AKU
Fabeha Pervez, Office of Communications, Aga Khan University
M.+92 333 350 8535 | E. [email protected]


