As CEPI ramps up vaccine research, a new study warns Lassa fever is set to expand its reach across Africa

Climate change and other environmental factors could cause Lassa fever to spread to additional regions within Africa, new research predicts.
CEPI is the leading funder of research into vaccines against the potentially deadly virus.
Up to 600 million people could be at risk of developing the potentially deadly Lassa fever in the future as a result of climate change and other factors, a new study conducted by scientific experts at Scripp Research and the University of Brussels has found.
After analysing environmental data looking at factors like temperature, rainfall, and presence of pasturelands, the study, published last month in Nature Communications, warned that the virus could extend its reach from West Africa (where it causes regular outbreaks) to also become a public health problem in other regions in Africa. Calculations on the number of people at risk also accounted for expected population growth in Africa over the next fifty years.
First identified in two nurses in the town of Lassa, in remote Nigeria, in 1969, Lassa fever is a haemorrhagic disease, caused by a virus of the same name. It is typically carried and spread by a species of infected rodent known as the Natal Multimammate rat (scientific name Mastomys natalensis).
Scanning electron micrograph of Lassa virus budding off a cell. Credit: NIAID
The pathogen has been on CEPI's target list since its launch in 2017, with the Coalition establishing itself as one of the largest funders of Lassa fever research. This latest study makes the case for the importance of CEPI's investments and our ambition to develop a Lassa vaccine for routine immunisation.
"Lassa fever has long been thought of as a disease solely affecting the West African region," CEPI's Director of Epidemiology, Gabrielle Breugelmans, explains. "But this study shows that this might not necessarily be the case in the future. The potent mix of changing climatic factors, human encroachment into previously remote areas, and population growth may create the perfect environment for this virus to spread into Central and East Africa- and we must be better prepared."
At present, hundreds of thousands of cases are estimated to occur annually across countries including Benin, Ghana, Guinea, Liberia, Mali, Nigeria, Sierra Leone, and Togo, however the true disease burden is unknown and likely much higher than current assessments. Although rare, in addition to transmission via infected rodents, the virus can also be spread through person-to-person contact when coming into direct contact with bodily secretions from an infected patient. For example earlier this year, three cases of Lassa fever were also reported in the UK following international travel and subsequent human-to-human transmission.
Outbreaks continue to occur with worrying regularity – and, as the new research shows, their suitable ‘impact zone' could increase in the future. The world urgently needs a vaccine against this potentially deadly pathogen.”

Credit: CEPI Enable partners in Benin
While the majority of patients will be asymptomatic, one in five will experience severe symptoms. These can range from mild headache and fever to vomiting, swelling of the face, pain in the chest, back and abdomen, and bleeding from body parts, including the eyes and nose.
On average, 1% of cases are fatal, with the disease especially risky for pregnant women in their third trimesters. In those that recover, hearing loss is commonly reported post-infection.
"No vaccines currently exist for human use" says Breugelmans "yet the warning signs are there for all to see. Outbreaks continue to occur with worrying regularity — and, as the new research shows, their suitable ‘impact zone' could increase in the future. The world urgently needs a vaccine against this potentially deadly pathogen."
Vaccines enter clinical trials
Fortunately, there has been major progress within Lassa fever vaccine research in recent years — with CEPI named as one of the top funders in this disease research in a report by the global thinktank Policy Cures Research.
A signification portion of this funding is being used to support the development of five Lassa vaccines. Four of these vaccine candidates—created by IAVI, Themis Bioscience, Inovio and Emergent Biosolutions—have now entered clinical trials, becoming some of the first in the world to assess the safety of Lassa vaccines in humans through Phase I studies.
CEPI will continue to support its Lassa vaccine partners and monitor progress as vaccine candidates make their way through clinical trials.
Healthy adult volunteers are participating in these studies at sites around the world. For example, last month IAVI and CEPI announced the launch of a Phase I trial in Liberia's capital, Monrovia, to assess the safety and tolerability of IAVI's Lassa vaccine candidate, with support from PREVAIL, a Liberia-US clinical research collaboration established by the U.S. National Institute of Allergy and Infectious Diseases (NIAID) and the Ministry of Health in Liberia. The study, which has already launched in the US, will offer participants at least one dose of the vaccine or placebo. Volunteers will be monitored for up to a year following vaccination.
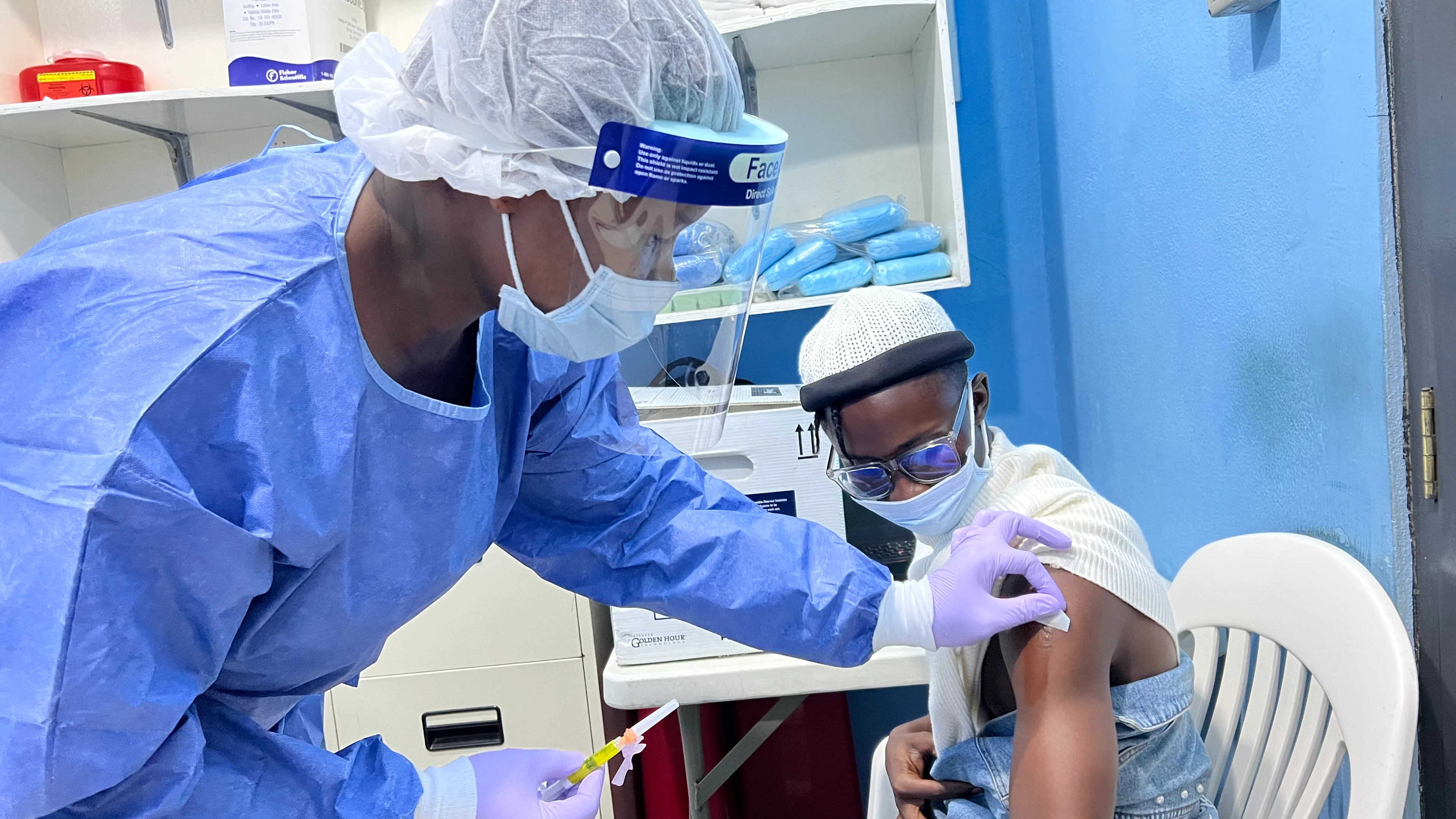
First participant vaccinated in a Phase 1 clinical study of the IAVI Lassa fever vaccine in the PREVAIL clinic, Redemption Hospital, Liberia. Credit: PREVAIL
A Phase I First-in Human trial was also launched last month by CEPI's partner Emergent Biosolutions to assess their own Lassa vaccine candidate. Here, up to 36 healthy adults will participate in the research at the Navrongo Health Research Centre and Kintampo Health Research Centre in Ghana.
Elsewhere in Ghana, our partners Inovio have recently completed a Phase Ib study for their Lassa vaccine candidate at Noguchi Memorial Institute for Medical Research. This is the first Lassa vaccine trial to be completed on the African continent. In total, 220 participants took part in the research, with enrollment and the trial itself having to be conducted around COVID-19 lockdowns.
CEPI's ultimate goal as part of its $3.5bn plan, launched last year to minimise or even eliminate future epidemic and pandemic threats, is to support the development of one or more Lassa vaccines through to licensure.
"As we work towards this milestone target over the coming years, CEPI will continue to support its Lassa vaccine partners and monitor progress as vaccine candidates make their way through clinical trials" explains CEPI's Director of Clinical Development, Jakob Cramer.
Tracking the true disease burden
Following successful completion of Phase I and later Phase II trials in the next few years, vaccine candidates will head into late-stage trials to assess their efficacy. This is where scientists will discover whether people in the clinical trial who got vaccinated with the Lassa vaccine were less likely to develop the disease compared to people who got the placebo shot.
Ahead of doing so, CEPI has initiated the largest-ever Lassa fever research programme, named Enable, to increase our knowledge of the disease burden across West Africa.
In total, CEPI is providing up to US $29 million in funding to support local partners in Benin, Guinea, Liberia, Nigeria, and Sierra Leone who have enrolled up to 23,000 participants to better understand the rate, location, and spread of Lassa virus across the region.

Credit: CEPI Enable partners in Guinea
Through a range of assessment types, including participants receiving home visits from health care workers or phone calls from the study team, the researchers hope that they can track more patients across the five countries who have mild disease but fail to seek diagnosis, as well as picking up those in remote regions where there are difficulties in accessing health care services for testing.
The study will also select a pool of participants to see whether they already have Lassa fever antibodies. If so, this would indicate that they have previously been infected and are protected against (immune from) the disease. Using these figures, researchers can also get an idea on the number of people who remain at risk of Lassa infection and therefore can benefit from Lassa vaccination.

Credit: CEPI Enable partners in Liberia
Data collected in the Enable partner countries—which will be made available on open-access publication sites for the benefit of the whole scientific community —will then be used to guide where and how these future late-stage efficacy trials to evaluate the CEPI-funded Lassa vaccines should take place. Enable data could also help to define future vaccination strategies when a Lassa vaccine has been approved for use for example by helping to identify priority populations at risk who should receive the vaccine first.
A collaborative effort
Altogether, eighteen partners (including CEPI) are involved in the strategic level planning or in-country implementation for Enable. This includes the Nigeria Centre for Disease Control (NCDC), a long-term partner to CEPI who were instrumental in the launch and running of the first ever Lassa fever conference, in 2019. NCDC are also leading the Nigerian component of the Enable study, alongside other in-country partners.
Later this month CEPI will co-host a workshop with NCDC and other Enable partners (from Benin, Guinea, Liberia, Nigeria, Sierra Leone) in Abuja, Nigeria to discuss the initial study results, while also checking up on progress since the 2019 conference. The meeting will be used as a platform for our researchers to build connections, and discuss their work, findings and scientific and technical priorities going forward with their peers. Meeting attendees will also discuss how the laboratory capabilities built in partner countries during our Enable study could be sustained when the research comes to an end and aligned with other Lassa and infectious disease surveillance systems and efforts across the region.
Our upcoming workshop provides the first in-person event co-hosted by CEPI and Nigeria CDC where our study partners can come together to share their experiences and study site progress.
Further details of plans for the workshop will be shared in the coming weeks.
"After much discussion and planning, our upcoming workshop provides the first in-person event co-hosted by CEPI and Nigeria CDC where our study partners can come together to share their experiences and study site progress, while also providing us with a platform to discuss gaps within the Lassa research field more widely" says Breugelmans.
This latest study warning of the threat of environmental factors on Lassa fever serves as a stark reminder on the need for these important events.
"The field is progressing, but there's still much more we need to do as a global research community to accelerate vaccine development efforts" outlines Cramer.
"The COVID-19 response has shown the power of working together, across teams, countries, and sectors, to tackle the pressing problem in front of you. We hope to follow a similar approach here at CEPI, with our partners, to build on our learnings to date and reach our ultimate end-goal of a licensed Lassa vaccine."