West Africa is one step closer to being protected against the deadly Lassa fever disease with IAVI’s vaccine candidate showing promise in the first stage of clinical testing.
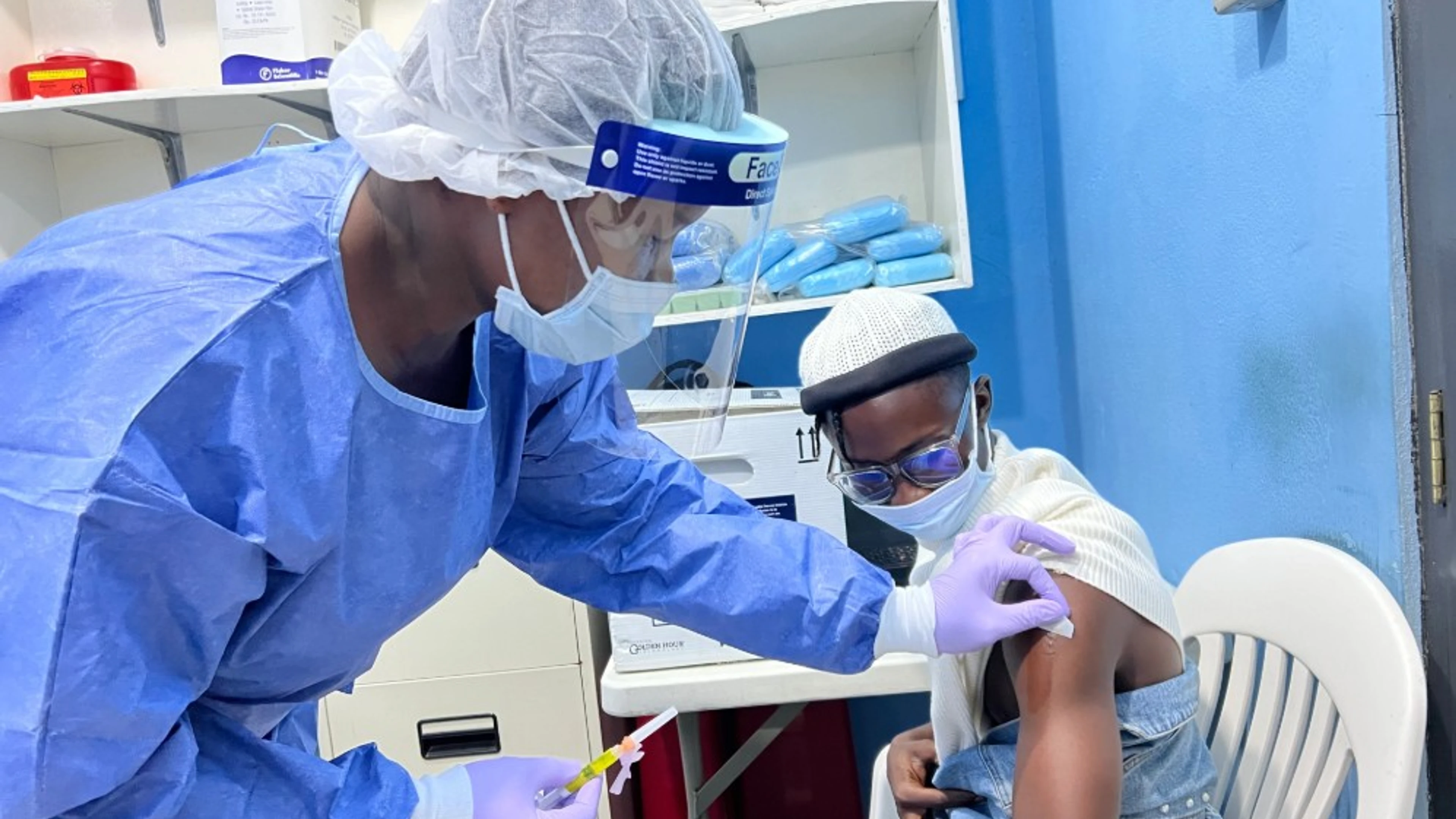
CEPI-backed research, published today in the New England Journal of Medicine, shows that over 100 healthy adults enrolled in a Phase I trial in the US and Liberia who received one dose of the Lassa fever vaccine candidate generated a robust and sustained immune response. Testing also found the vaccine candidate, known as rVSVΔG-LASV-GPC, has an acceptable safety profile.
rVSVΔG-LASV-GPC uses the same vaccine technology ‘backbone’ as the licensed Merck Ebolavirus vaccine, ERVEBO.
“These encouraging results add to a growing body of evidence that demonstrates the safety and immunogenicity of IAVI’s single-dose Lassa vaccine candidate” says Dr Swati Gupta, Vice President and Head of Emerging Infectious Diseases and Epidemiology at IAVI.
A Phase II trial, supported by CEPI, has now commenced across Nigeria, Sierra Leone and Ghana, making rVSVΔG-LASV-GPC the most advanced Lassa fever vaccine candidate in clinical testing. The latest research study aims to generate additional evidence on the performance of the Lassa fever vaccine in a larger set of volunteers.
Lassa fever is widespread across West Africa, with annual outbreaks affecting hundreds of thousands of people from November through to May. The virus is primarily spread by infected rodents known as Mastomys rats, although infections can also pass between people, especially in healthcare settings.
Most people are thought to be asymptomatic, however some of those infected can suffer from more severe symptoms including vomiting, swelling, chest pain and bleeding from multiple body parts including the eyes and nose. Infection can prove fatal in some cases and pregnant women can suffer from severe complications for both mother and baby.
While a known threat to West Africa, there is concern that up to 600 million people could be at risk of Lassa fever by 2050 on the African content because of the changing climate and increased movement of people.
CEPI is one of the world’s leading funders of Lassa fever research. We are working with partners in West Africa and around the world to prepare for future outbreaks by advancing one or more Lassa fever vaccines to licensure.
“The promising Phase 1 data for IAVI’s vaccine candidate takes us one step closer towards a much-needed Lassa fever vaccine which, if successful, could save thousands of lives and avert millions of dollars of societal costs in the West African countries that bear the burden of this disease” says Dr Kent Kester, Executive Director of Vaccine R&D at CEPI.
News of the positive vaccine findings follows the historic endorsement of a communiqué at the Lassa International Conference in September 2025, where West African Ministers of Health reaffirmed their commitment to advance a Lassa fever vaccine to licensure.
The communiqué recognises the need to get an affordable Lassa fever vaccine to the people who need it most across the region and highlights how the programme of work, led by the Lassa fever Coalition, is a pioneering cross-country effort and serves as a cornerstone of pandemic preparedness for the region. Regional leaders committed to supporting the development of IAVI’s Lassa vaccine candidate through a collaborative co-funding approach and joint action to mobilize and secure resources through advocacy and regional coordination.


.webp)
