Global consortium plans coordinated human challenge studies in hunt for transmission-blocking coronavirus vaccines
- $57 million project will test inhaled and nasal vaccines designed to stop viral infection
- Human challenge studies uniquely able to progress such transmission-blocking vaccines
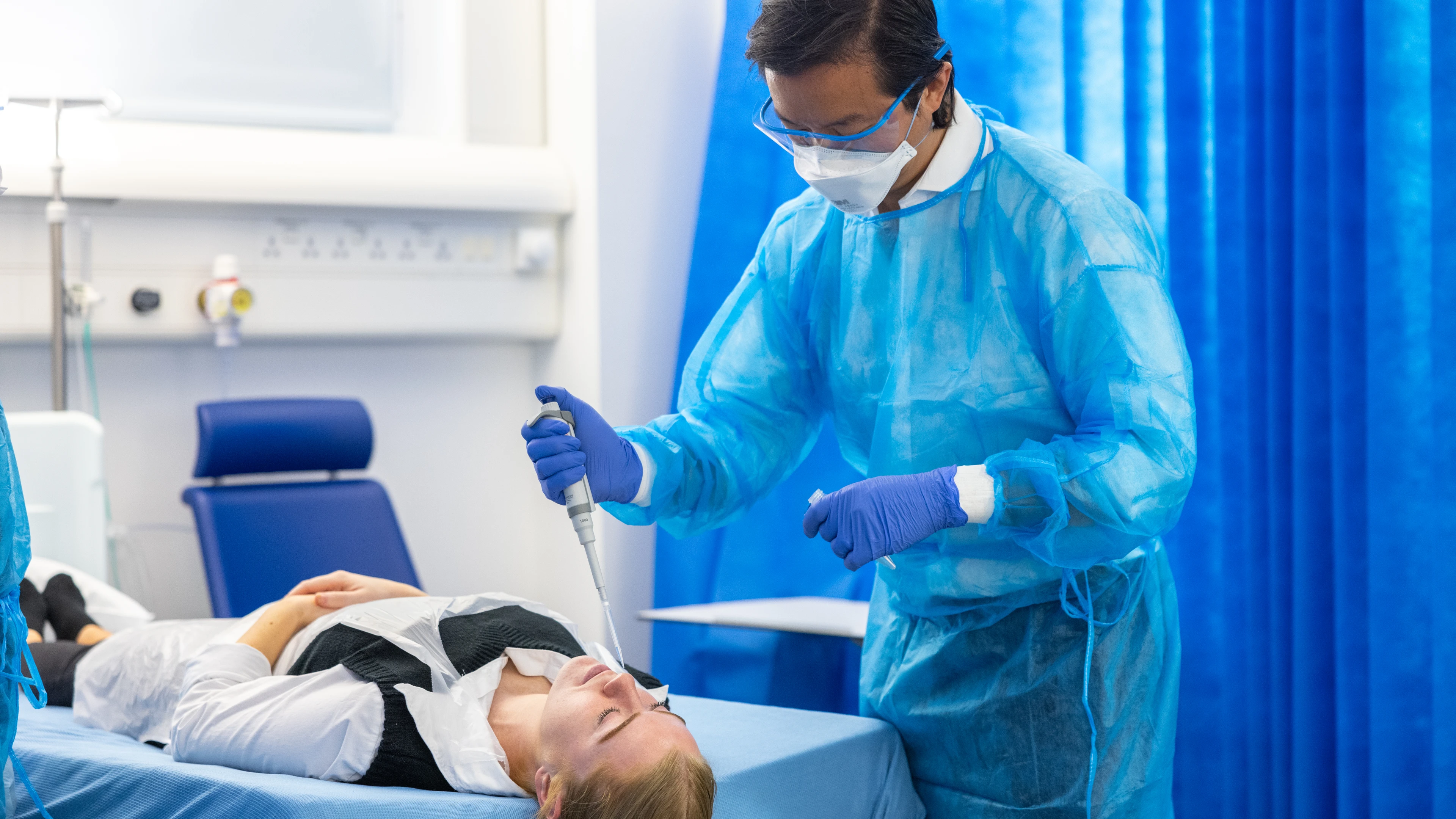
- Coordinated trials involve safely inducing infection in small groups of healthy volunteers
OSLO/LONDON, March 11, 2024 - An international consortium of researchers specialising in human challenge studies is embarking on a US$57 million project to develop advanced, virus-blocking coronavirus vaccines that could stop SARS-CoV-2 and other coronaviruses from infecting people in the first place.
Led by Imperial College London and co-funded by the European Union’s Horizon Europe Programme and CEPI, the Coalition for Epidemic Preparedness Innovations, the consortium of more than a dozen scientific teams and organisations will begin by running trials to select particular viruses and identify the best conditions under which to safely induce infection in healthy volunteers. A human challenge study is a carefully managed medical research study, during which volunteers are intentionally given an infection in a safe way, with healthcare support.
Researchers at multiple clinical research facilities will then use a selected virus to try to infect healthy volunteers who have received an experimental vaccine. Unlike traditional vaccines which are injected into muscle, these experimental vaccines will be inhaled into the lungs or sprayed in the nose and are designed to induce a type of protection known as mucosal immunity – which scientists believe could be the key to stopping onward transmission of coronaviruses.
“Vaccines that can stop transmission of a virus, rather than only reducing the severity of the disease it causes, are crucial to being able to end pandemics and epidemics swiftly,” said Dr Richard Hatchett, CEPI’s Chief Executive Officer. “If we could find a way to induce virus-blocking mucosal immunity with the next generation of COVID-19 vaccines, for example, we could then dramatically reduce the circulation of the SARS-CoV-2 virus andhence limit its ability to generate dangerous new variants.”
Human challenge studies are unique in their ability to investigate and understand the onset and development of disease in a safe and highly controlled environment. They enable scientists to observe and analyse complex interactions between viruses and the human immune system and to identify ways to disrupt and block viral infections.
The five-year Mucosal Immunity in human Coronavirus Challenge (MusiCC) project will be led by Imperial College London, whose specialist researchers have built up years of experience in using human challenge studies to deepen scientific understanding of a range of infectious diseases. In February 2021, Imperial ran the world’s first human challenge study for COVID-19 and currently leads the world’s first human challenge study for non-typhoidal salmonella.
Chris Chiu, Professor of Infectious Diseases at Imperial College London and principal investigator for MusiCC, said: “Coronaviruses typically infect people through cells lining their nose, throat and lungs. Mucosal immunity generated at these surfaces is highly specialised and very different to immune responses in the circulation. Since it directly acts in the place that viruses enter and exit the body, it could be the key to developing vaccines that can block viruses from being able to spread from one person to another.”
“This exciting project enables us to bring together world class expertise in human challenge studies. With our partners, we will build on the fundamental groundwork we’ve already carried out at Imperial, and through our leading expertise and experience in human challenge studies for a range of pathogens, help to develop the next generation of transmission-blocking vaccines.”
As the lead and convener of the consortium, Imperial will work with all MusiCC partners, including, for example, the University of Antwerp’s Vaccinopolis in Belgium, to establish human challenge models that can be used in multiple trial sites. The trials will test potential mucosal vaccine candidates against betacoronaviruses – the sub-family of coronaviruses that includes the SARS-CoV-2 virus that causes COVID-19, several seasonal viruses that cause common colds, and the MERS coronavirus that causes Middle East Respiratory Syndrome.
Using harmonised standard operating procedures, the trials will take place across several sites in the UK, Europe, the United States and Singapore and will each involve a small group of young, healthy volunteers. In the challenge trial, volunteers will first receive either a dose of an investigational vaccine designed to provide mucosal coronavirus immunity or placebo before being intentionally exposed to a calibrated dose of SARS-CoV-2. A model using a seasonal coronavirus called OC43 is also being developed for similar use.
The project’s launch follows the Call for Proposals issued by CEPI and co-funded by the European Union to support international research conducting human challenge-based vaccine trials that seek to improve scientific understanding of mucosal immunity against Betacoronaviruses. CEPI and its partners are committed to enabling equitable access to the outputs of this project in line with CEPI’s Equitable Access Policy. This includes a commitment that any vaccines developed are made available first and at an affordable price to the most vulnerable populations. Data generated by MusiCC will be published with open access for the benefit of the global scientific community.
The European Commission’s Laurent Muschel, Head of HERA, and Marc Lemaître, Director-General for Research and Innovation said: “Research and innovation is an essential part of the jigsaw when it comes to health emergency and pandemic preparedness. Supported through up to €35 million by the European Union’s Horizon Europe programme, MusiCC is an exciting and potential game-changing project, opening up the possibility to target and block viruses, stopping their transmission. Investing in research for health emergency preparedness and response remains a priority for the European Commission to protect the health of our citizens and public health beyond Europe.”
Professor Hugh Brady, President of Imperial College London, said: “I’m delighted to see Imperial’s Professor Chris Chiu leading this consortium. Working with our global partners, we will be able to use human challenge studies to help develop future vaccines that will benefit humanity. This work is a fantastic example of Imperial’s ability to bring people and organisations together to create real-world impact.”
ENDS
Notes to Editors
About CEPI
CEPI was launched in 2017 as an innovative partnership between public, private, philanthropic and civil organisations. Its mission is to accelerate the development of vaccines and other biologic countermeasures against epidemic and pandemic threats so they can be accessible to all people in need. CEPI has supported the development of more than 50 vaccine candidates or platform technologies against multiple known high-risk pathogens or a future Disease X. Central to CEPI’s pandemic-beating five-year plan for 2022-2026 is the ‘100 Days Mission’ to compress the time taken to develop safe, effective, globally accessible vaccines against new threats to just 100 days.
About Imperial College London
Imperial College London is a global top ten university with a world-class reputation. Imperial’s 22,000 students and 8,000 staff are working to solve the biggest challenges in science, medicine, engineering and business.
Imperial ranks sixth in the 2024 QS World University Rankings and eighth in the 2024 Times Higher Education World University Rankings. The 2021 Research Excellence Framework (REF) found that it has a greater proportion of world-leading research than any other UK university. It also received a Gold Award in the 2023 Teaching Excellence Framework (TEF). Imperial was named University of the Year in the Daily Mail University Guide 2024, University of the Year for Graduate Employment in The Times and Sunday Times Good University Guide 2024, and awarded a Queen’s Anniversary Prize for its COVID-19 response.
Imperial has an extensive track record in infection research and experimental medicine studies to understand how infectious agents cause disease in human beings. As a world-leader in the specialised experimental medicine methods represented by human challenge (or controlled human infection), teams at Imperial have led their development to understand how disease occurs, how the immune system protects us from infection, and to test new vaccines and treatments. Such studies have involved pathogens as diverse as malaria, bacteria including salmonella, and respiratory viruses. This track record resulted in Imperial’s appointment by the UK Vaccines Taskforce to lead the SARS-CoV-2 human challenge consortium during the COVID-19 pandemic, which has led to a programme of follow-on studies to understand how SARS-CoV-2 causes disease and how to harness the immune system to improve outcomes following infection. https://www.imperial.ac.uk/
About Horizon
Horizon Europe — #HorizonEU — is the European Union's flagship Research and Innovation programme, part of the EU-long-term Multiannual Financial Framework (MFF) with a budget of EUR 95,5bn to spend over a seven-year period (2021-2027). Under Horizon Europe, health research will be supported with the aim to find new ways to keep people healthy, prevent diseases, develop better diagnostics and more effective therapies, use personalised medicine approaches to improve healthcare and wellbeing, and take up innovative health technologies, such as digital ones.
About Health Emergency Preparedness and Response Authority (HERA)
HERA’s mission is to strengthen Europe's ability to prevent, detect, and rapidly respond to cross-border health emergencies, by ensuring the development, manufacturing, procurement and equitable distribution of key medical countermeasures. Created in the aftermath of the COVID-19 pandemic to replace ad hoc solutions to pandemic management and response, it is a key pillar of the European Health Union and a fundamental asset to strengthen the EU's health emergency preparedness and response.


.webp)
