CEPI funding call launches to rapidly generate additional clinical research on COVID-19 vaccines
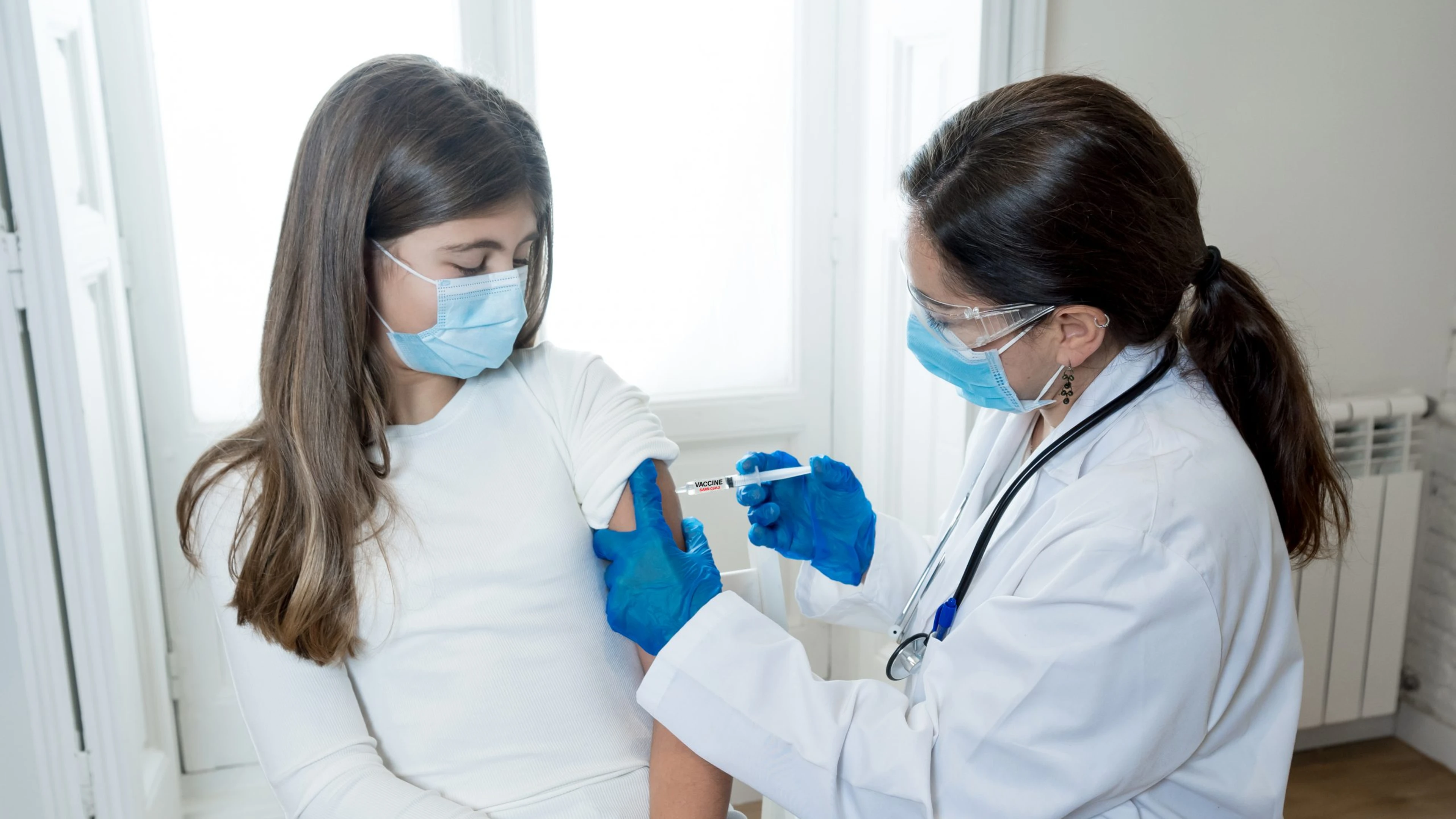
Launched today, the new CEPI Call will provide up to US $140 million in funding to vaccine developers and other research institutions for rapid complementary clinical data on COVID-19 vaccines.
Deliberately kept broad in scope, examples of scientific programmes to be supported through the Call include clinical trials in pregnant women, infants and children, and immunocompromised populations, as well as studies on booster doses, length of vaccine efficacy, ‘mix and match' strategies, and dosing intervals.
Purpose of Call is to fill in current clinical research and development gaps to expand access to COVID-19 vaccines.
As part of their proposal, applicants must show plans to increase equitable access to COVID-19 vaccine being assessed.
28 January 2021, Oslo, Norway — CEPI, the Coalition for Epidemic Preparedness Innovations, has today opened a new funding opportunity to support the rapid generation of additional clinical research on COVID-19 vaccines. Complementary to ongoing vaccine R&D efforts, the purpose of the new Call is to provide critical evidence to address current gaps in our clinical knowledge of vaccine performance both now and in the long-term, in order to expand access to COVID-19 vaccines as part of the global vaccination rollout.
Up to US $140 million, contingent upon the availability of funds, is to be made available to scientific groups worldwide to carry out the supporting research on COVID-19 vaccines either already available to populations in some countries under ‘emergency use approval' or ‘conditional licensure' or similar, or currently in clinical development. This includes COVID-19 vaccine developers, as well as public health organisations, academic institutions and others who may not be directly carrying out their own COVID-19 vaccine development but have the capacity, ability and partnerships established to carry out research on a chosen vaccine. As one of the global health organisations co-leading the response to the COVID-19 pandemic, CEPI is launching this Call to ensure expanding access to COVID-19 vaccines remains a global priority and to also act in its facilitating role, connecting successful applicants to initiatives and projects supported by CEPI, to advance their clinical research.
Filling in clinical R&D gaps
Over the course of 2020, the world has seen incredible and historic progress in vaccine development and deployment, with multiple COVID-19 vaccines being rolled out in adult populations in some nations following successful regulatory review, countries preparing to launch their own vaccination programmes through COVAX—the global initiative co-led by CEPI, Gavi, the Vaccine Alliance, and the World Health Organization (WHO) to develop, manufacture, and enable equitable global access to 2 billion doses of safe and effective COVID-19 vaccines by the end of 2021—and further vaccine candidates undergoing clinical testing.
Despite these valiant efforts, a number of critical clinical research and development gaps—identified at a recent WHO consultation meeting held 15 January 2021 (link pending)—remain and need to be urgently addressed in order to expand access to COVID-19 vaccines. Gaps in global COVID-19 vaccine data are also perpetuated by the emergence of novel variants around the world, highlighting the need to continue to invest in vaccine R&D.
The scope of research to be funded through this new CEPI opportunity, open through to May 28, 2021, has therefore been deliberately kept broad to quickly generate a range of new relevant data, in addition to evidence already produced or being produced through core clinical assessment (such as Phase I safety trials and Phase II/III efficacy trials).
For example, programmes supported through this Call could advance current COVID-19 immunisation efforts through providing data on demographics and age-groups that may not currently be eligible to receive doses of COVID-19 vaccines, like pregnant and lactating women, infants and children, and those who are immunocompromised. If trial assessments indicate COVID-19 vaccines are safe, tolerable and produce an immune response in such populations, data could inform and influence current vaccination rollout strategies around the world to expand access to these key groups. Clinical studies in some age-groups like infants and children could also provide important information on the size of vaccine dose needed to be impactful. In addition, transmission studies, whereby research is conducted looking at the impact of a COVID-19 vaccine to prevent the virus from ‘spreading' from one individual to another, would be supported as sufficient evidence is not yet available.
Looking ahead, the Call is also open to applicants wishing to provide data that could guide future vaccination efforts, for example whether booster doses may be necessary; the length of time a vaccine remains effective (the degree to which a vaccine prevents disease, and possibly also transmission); and the potential impact of novel variants on vaccine performance. Such forward-looking research will be important as global health experts agree that COVID-19 will become endemic, circulating in populations in the future, and eventual global protection against COVID-19, provided through vaccination programmes, will therefore need to be upheld.
Other potential focal points of interest which could be funded under this Call include research programmes which aim to identify reliable markers of COVID-19 immunity (also referred to as correlates of protection). With some nations looking to extend the interval between the administration of two-dose regimens of COVID-19 vaccines in order to increase the number of individuals receiving their first dose of a given vaccine, and/or deploy a mixture of vaccines to increase use of available stock and speed up mass vaccination, the funding opportunity may also support researchers wishing to provide additional data on dose-timing and ‘mix and match' strategies.
Fast data to rapidly expand global access
In order to have maximal effect in responding to the COVID-19 pandemic as quickly as possible, applicants should be able to begin their research programmes within three to six months following successful review, with at least interim data to be produced and shared widely before the end of 2021 to inform the ongoing vaccination rollout.
As CEPI is committed to the principle of global equitable access, applicants must demonstrate how equitable access to COVID-19 vaccines will be better enabled through their proposed work. For example, applications seeking to carry out clinical studies in low-income and middle-income countries are encouraged so that vaccines can be confidently rolled out in such locations following testing and approval. Proposals could also highlight the applicant's intention to make the COVID-19 vaccine, studied as part of this Call, available through the COVAX Facility to all countries participating in the global initiative. Without consideration of plans to expand equitable access to their COVID-19 vaccine, applicants will not receive funding.
With multiple safe and effective vaccines beginning to be deployed around the world, we are now building the tools to get us out of this unprecedented crisis.
However, we must ensure that we continue to generate the crucial evidence to fill in current clinical research and development gaps and understand how these vaccines and others under development can support special populations, respond to novel variants, influence virus transmission, and prepare us for future vaccination programmes, for example if there is a need for booster doses.
The noteworthy phrase frequently stated throughout the course of this devastating crisis is ‘no one is safe until we are all safe'. Together with other ongoing studies around the world, additional evidence supplied through this Call could help expand access to COVID-19 vaccines and ensure as many people as possible can be protected from this dreadful virus. It's through these collective response efforts that we hope to begin to see societies, economies and our livelihoods restored.
Apply online
The Call for funding is now open on the CEPI website, with applicants invited to submit an Expression of Interest, including a description of the current status of the COVID-19 vaccine candidate and an overview of plans for the clinical programme.
Each Expression of Interest will be evaluated on a rolling basis by CEPI vaccine R&D experts and external reviewers with respect to: addressing current gaps in clinical knowledge relating to the specific COVID-19 vaccine; the potential impact of the additional data generated through the research in advancing global efforts to end the COVID-19 pandemic; equitable access provisions; and against the current CEPI COVID-19 portfolio.
—ENDS—
Additional details
All information relating to the Call, including full scope of research projects that could be supported through funding, complete eligibility and review criteria and details on how to apply is available on the CEPI ‘Calls for Proposals' webpage, under ‘Calls for Proposals — Complementary clinical trials: Expanding access to COVID-19 vaccines and rapid response to clinical development gaps'.
Clinical development gaps were identified in the WHO Consultation COVID-19 Vaccines — Knowledge Gaps and Research Priorities held on 15 January 2021. Proposed studies may be new ideas or an amendment to existing clinical trial plans. It is not the intent of this Call for Proposals to support clinical trials already included in the vaccine developer's core Clinical Development Plan supporting initial emergency use approval / (conditional) licensure or similar (for example initial Phase 1 dose / formulation finding trials or pivotal Phase 2/3 vaccine efficacy trials).
Applicants must demonstrate a plan to increase access to COVID-19 vaccine in their proposal. An access plan can include commitments and strategies to enable access to vaccines as well as more concrete elements. Access plans need to facilitate availability, accessibility, and affordability for people in low- and middle-income countries ie, commitments to the Gavi COVAX Advance Market Commitment, registration commitments, equitable pricing strategies, sufficient supply commitments, WHO prequalification etc.
In addition to indicating plans to support and expand equitable access to their COVID-19 vaccine, applicants are strongly encouraged to use international reference standards to assess the immune response elicited by their COVID-19 vaccine candidates where possible and to consider plans to integrate their immunological testing with CEPI's Centralised Laboratory Network. Eligibility criteria will also consider participation in the meta Data Safety and Monitoring Board (mDSMB) scheme offered as part of the Safety Platform for Emergency vACcines (SPEAC) project.
The Call is open for Expressions of Interest from 28 January 2021 to 28 May 2021 (15.00 CET) and may be extended or amended depending on need and external situation at any point. Responses to applicants will be issued as soon as available.
About CEPI
CEPI is an innovative partnership between public, private, philanthropic, and civil organisations, launched at Davos in 2017, to develop vaccines to stop future epidemics. CEPI has moved with great urgency and in coordination with WHO in response to the emergence of COVID-19. CEPI has initiated 11 partnerships to develop vaccines against the novel coronavirus. The programmes will leverage rapid response platforms already supported by CEPI as well as new partnerships. The aim is to advance COVID-19 vaccine candidates into clinical testing as quickly as possible.
Before the emergence of COVID-19 CEPI's priority diseases included Ebola virus, Lassa virus, Middle East Respiratory Syndrome coronavirus, Nipah virus, Rift Valley Fever virus and Chikungunya virus. CEPI also invested in platform technologies that can be used for rapid vaccine and immunoprophylactic development against unknown pathogens (Disease X).
Follow our news page for the latest updates. Follow us on Twitter and LinkedIn.
About COVAX
COVAX, the vaccines pillar of the Access to COVID-19 Tools (ACT) Accelerator, is co-led by CEPI, Gavi and WHO — working in partnership with developed and developing country vaccine manufacturers, UNICEF, the World Bank, and others. It is the only global initiative that is working with governments and manufacturers to ensure COVID-19 vaccines are available worldwide to both higher-income and lower-income countries.
CEPI is leading on the COVAX vaccine research and development portfolio, investing in R&D across a variety of promising candidates, with the goal to support development of three safe and effective vaccines which can be made available to countries participating in the COVAX Facility. As part of this work, CEPI has secured first right of refusal to potentially over one billion doses for the COVAX Facility to a number of candidates, and made strategic investments in vaccine manufacturing, which includes reserving capacity to manufacture doses of COVAX vaccines at a network of facilities, and securing glass vials to hold 2 billion doses of vaccine. CEPI is also investing in the ‘next generation' of vaccine candidates, which will give the world additional options to control COVID-19 in the future.
Gavi is leading on procurement and delivery for COVAX, coordinating the design and implementation of the COVAX Facility and the COVAX AMC and working with Alliance partners UNICEF and WHO, along with governments, on country readiness and delivery. The COVAX Facility is the global pooled procurement mechanism for COVID-19 vaccines through which COVAX will ensure fair and equitable access to vaccines for all 190 participating economies, using an allocation framework formulated by WHO. The COVAX Facility will do this by pooling buying power from participating economies and providing volume guarantees across a range of promising vaccine candidates. The Gavi COVAX AMC is the financing mechanism that will support the participation of 92 low- and middle-income countries in the Facility, enabling access to donor-funded doses of safe and effective vaccines. UNICEF and the Pan-American Health Organisation (PAHO) will be acting as procurement coordinators for the COVAX Facility, helping deliver vaccines to all participants.
WHO has multiple roles within the COVAX: among other things it supports countries as they prepare to receive and administer vaccines and does so in partnership with UNICEF. It provides normative guidance on vaccine policy, regulation, safety, R&D, allocation, and country readiness and delivery. Its Strategic Advisory Group of Experts (SAGE) on Immunization develops evidence-based immunization policy recommendations. Its Emergency Use Listing (EUL)/prequalification programmes ensure harmonized review and authorization across member states. It provides global coordination and member state support on vaccine safety monitoring. It developed the target product profiles for COVID-19 vaccines and provides R&D technical coordination. Along with COVAX partners, it is developing a no-fault compensation scheme for indemnification and liability issues. COVAX is part of the Act accelerator which WHO launched with partners in 2020.
Media Contacts
CEPI
Email: [email protected]
Phone: +44 7387 055214


