Efforts to advance Rift Valley fever vaccines progress with new CEPI partnership
August 6 2021, Oslo, Norway, Greensboro, North Carolina, USA, and Entebbe, Uganda—The Coalition for Epidemic Preparedness Innovations (CEPI) has signed an implementing partner agreement with Integrum Scientific (USA) as part of its ongoing efforts to combat Rift Valley fever (RVF)—one of its priority diseases with epidemic potential.
Under the new partnership, serum material donated from patients recovered from RVF will support the development of an International Antibody Standard, a reference tool used when assessing the immune response induced by RVF vaccines undergoing clinical trials. The programme of work will also enable the calibration and validation of vaccine tests (known as "assays") so that accurate and standardised results on the immunogenicity of different RVF vaccines can be produced.
Integrum Scientific is now collecting serum from RVF survivors' across sites in Uganda through a partnership with the Uganda Virus Research Institute (UVRI) and will continue through to September 2021. Uganda has previously experienced RVF outbreaks.
CEPI will provide up to a total of US $142,000 in funding to support the work. The programme will contribute to CEPI's $3.5bn plan, launched in March, 2021, to tackle future epidemics and pandemics, which includes advancing vaccine candidates against known high-risk pathogens such as RVF.
Rift Valley fever: a disease with epidemic potential
First identified in 1931 during an investigation into an epidemic among sheep on a farm in the Rift Valley of Kenya, RVF is caused by an arbovirus that affects both animals and humans. The virus is transmitted by mosquitoes and blood-feeding flies, with most human infections resulting from contact with the blood or organs of infected animals or from the bites of an infected mosquito.
While most human cases are relatively mild, causing death in less than 1 in every 100 people it infects, a small percentage of patients can develop a much more severe form of the disease, including the haemorrhagic form where the fatality rate is as high as 50%.
The disease typically affects people living in pastoral communities. Most recently, outbreaks of RVF have been reported in Sudan, South Africa, and Kenya, however cases have been reported across sub-Saharan Africa—including Uganda—as well as the Arabian Peninsula.
With human activities, ecological change and climatic factors meaning that RVF has the potential to become more widespread, the World Health Organization (WHO) has classified RVF as a priority pathogen in urgent need of investment on its R&D Blueprint. With no vaccines approved for human use, CEPI extended its list of target diseases to include RVF in January 2019. To date, CEPI has supported two programmes with Wageningen University and Colorado State University to advance human RVF vaccines. Both candidates are currently in preclinical phases of vaccine development.
The role of survivor antibodies in creating new vaccines
In order to accurately assess the performance of RVF vaccine candidates, tools and tests which can infer levels of immune response induced by vaccines need to be developed and validated.
To start this work, Integrum Scientific, alongside UVRI, are recruiting patients who have recovered from RVF virus infection. With informed consent, recovered patients donate samples of their serum which contain antibodies—produced as part of the immune response to fight off prior infection with RVF virus—to the research. Serum material will be collected at multiple sites in different geographical regions in Uganda to provide a diverse pool of participants who may have been affected by different RVF virus strains. Serum will also be collected from healthy donors (who have not had prior RVF infection) to provide a basis for comparing results.
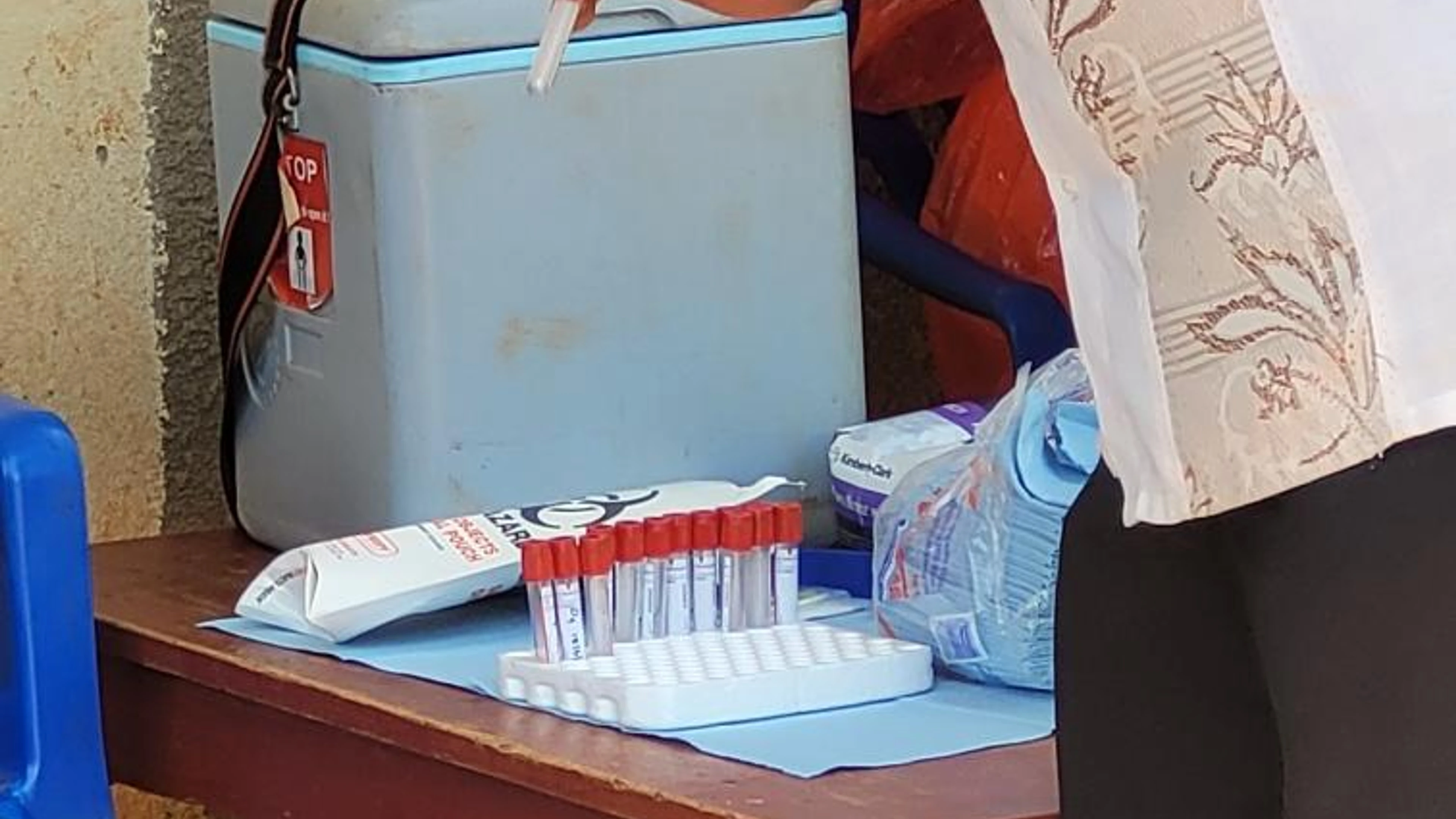
Credit: Integrum Scientific
Donated material will be tested and further processed at the National Institute for Biological Standards and Control (NIBSC) in the UK, under a partnership with CEPI to support the development of International Antibody Standards against multiple pathogens. Samples will be assessed to identify the individuals with the highest level of antibodies (i.e., the highest natural immune response) against RVF virus to establish an International Antibody Standard - a tool developed to act as a set reference point to assess whether a vaccine under clinical evaluation is likely to be a ‘good' vaccine (inducing a sufficient immune response in vaccinated individuals in comparison to those who have recovered from infection)*.
The serum material supplied by participants with lower antibody levels will also be used for the calibration and validation of vaccine tests, known as ‘assays', to detect a broad spectrum of antibody levels (from low to high) induced by a vaccine in clinical trials. These will be shared with developers and laboratories worldwide to help inform vaccine developers whether the level of immune response elicited by their RVF vaccine candidate is sufficient to progress to further testing.
Given the potential for Rift Valley fever to cause significant disruption to health, economies and societies across sub-Saharan Africa and other regions, we need to develop a suite of response tools, including vaccines, to ensure we are well prepared for future outbreaks.
However, if we are to successfully fight against the disease, it is paramount that we do not just directly support vaccine development programmes but also the creation and validation of key tools and tests which will be essential in measuring vaccine performance.
Our work with Integrum Scientific will therefore lead the way forward in creating antibody standards and calibrating assays so that scientists around the world can successfully assess future Rift Valley fever vaccine candidates. We express our thanks to the crucial work and support of Uganda Virus Research Institute, as well as the recovered Rift Valley fever patients for donating their serum, enabling us to progress these efforts.
Integrum is proud to be part of this effort with CEPI, the NIBSC, and the UVRI. Our team has a passion for outbreak readiness and response. We partner with local populations around the globe to reduce the impact of infectious disease outbreaks, especially in the most vulnerable regions of the world.
Our current Infectious Disease Scientific Advisory Board members came together from different organizations during the height of the 2014-2016 Ebola outbreak to develop a system to collect Plasmapheresis in West Africa. The effort provided hope to communities that had been devastated by the disease. Many survivors came early in the morning and stood by to give the ‘gold in their blood,' as they often referred to it, to help their countrymen.
Many had lost most of their family members and came from as far as 150 miles away to help make a real difference. We see the RVF project as a continuation of that important work, providing RVF virus survivors an opportunity to help protect future generations worldwide from the ravages of this disease.
We are quite excited to participate in this work with Integrum Scientific and CEPI. Rift Valley fever disease is now known to be continually circulating and causing disease in many parts of Uganda. It is one of the neglected zoonotic diseases. In the last couple of years, Rift Valley fever has been confirmed in several outbreaks in humans in Uganda.
Unfortunately, it affects people in the rural areas where it is not properly diagnosed and where most fevers are treated as malaria. People lose their lives because of lack of a vaccine. We are happy to be part of a partnership that is advancing the production and safe use of Rift Valley fever vaccines.
For several years, we have been involved in studies of survivors of Viral Haemorrhagic Fevers (VHFs) and have contributed to understanding the immune responses in survivors of these diseases. Through these constant contacts with the survivors we have established a relationship for continued studies, and I am confident that the survivors are also happy to contribute to the noble cause of using their blood to provide an antibody standard for use globally.
* Developed and in use for other diseases, an antibody standard is a pool of serum or plasma from multiple patients who have recovered from virus infection with high levels of antibodies produced as part of their natural immune response to the infection. The International Antibody Standard is a tool to calibrate those kits or tests used to determine the amount of antibodies in an individual, so that the antibody responses to vaccines tested from different developers using different kits or methods can be compared. This not only allows for the comparison between RVF vaccine candidates, but it is instrumental to determine a minimum level of antibody level required to protect from disease. In support of CEPI's ongoing commitments to equitable access, the aim is for global distribution of the International Antibody Standard, once established by WHO's Expert Committee on Biological Standardization (ECBS), to laboratories for accurate and harmonised assessment of RVF vaccine candidate performance.
—ENDS—
Further information about the partnership
Integrum Scientific was selected to complete the project following CEPI's Request for Proposals, launched July 2020, seeking individual actors or consortiums to source sera from recovered RVF patients.
Patients donating serum material to the research programme must have had a formal diagnosis of RVF. All participants must have signed an Informed Consent Form, outlining that samples will be exported to the NIBSC in the UK for testing and that analysis and the materials relating to the International Antibody Standard are intended for global distribution on a non-commercial basis to all vaccine developers and relevant testing laboratories which require an International Antibody Standard. A Material Transfer Agreement with the NIBSC enables international export and non-commercial global use of the International Antibody Standard that will be developed from this donated serum material. Integrum Scientific will screen patients to select suitable donors. CEPI's Product Profile further defines the necessary characteristics for the sourcing of serum material from recovered RVF patients.
About Rift Valley fever and Rift Valley fever vaccine programmes
Information on the symptoms and epidemiology of Rift Valley fever is available on the Priority Diseases section of the CEPI website.
CEPI currently has two RVF vaccine candidates in its vaccine portfolio — (i) with Wageningen Bioveterinary Research, part of Wageningen University & Research providing up to US $12.5 million for vaccine manufacturing, preclinical research, and a Phase I study to assess the safety, tolerability, and immunogenicity of a single-dose vaccine candidate (RVFV-4S) against RVF virus for use in humans, and (ii) with Colorado State University providing up to US $9.5 million for manufacturing and preclinical studies to assess a single-dose vaccine candidate against RVF. Funding is provided by CEPI with support from the European Commission's Horizon 2020 programme.
About CEPI
CEPI is an innovative partnership between public, private, philanthropic, and civil organisations, launched at Davos in 2017, to develop vaccines against future epidemics. Prior to COVID-19 CEPI's work focused on developing vaccines against Ebola virus, Lassa virus, Middle East Respiratory Syndrome coronavirus, Nipah virus, Rift Valley Fever virus and Chikungunya virus - it has over 20 vaccine candidates against these pathogens in development. CEPI has also invested in new platform technologies for rapid vaccine development against unknown pathogens (Disease X).
During the current pandemic, CEPI initiated multiple programmes to develop vaccines against SARS-CoV-2 and its variants with a focus on speed, scale, and access. These programmes leverage the rapid response platforms developed by CEPI's partners prior to the emergence of COVID-19 as well as new collaborations. The aim is to advance clinical development of a diverse portfolio of safe and effective COVID-19 candidates and to enable fair allocation to these vaccines worldwide through COVAX.
CEPI's 5-year plan lays out a $3.5 billion roadmap to compress vaccine development timelines to 100 days, develop a universal vaccine against COVID-19 and other Betacoronaviruses, and create a "library" of vaccine candidates for use against known and unknown pathogens. The plan is available at endpandemics.cepi.net.
Follow our news page for the latest updates. Follow us on Twitter and LinkedIn.
About Integrum Scientific
Integrum Scientific is an Outbreak Readiness and Response company founded to improve the effectiveness of global and local clinical research on infectious diseases. We aim to improve disease surveillance, increase prevention, standardize preparation, and expedite response times. We believe infectious disease preparedness is not only a national security priority but a global humanitarian imperative. To ensure we are ready for the next outbreaks, Integrum seeks to increase and improve the training and education available to local healthcare workers and researchers, expand the quality and quantity of local labs and their resources, and expand clinical research infrastructures to ensure relief can reach the hardest hit areas faster. To accomplish this goal, we seek to partner with academia, government organizations, non-governmental organizations, and industry.
Our medical and scientific researchers, infrastructure and logistics experts, and communication specialists bring decades of on-the-ground experience working on some of the most critical global health challenges in the most remote regions of the world. Our scientists and clinical researchers have conducted ground-breaking research and landmark trials throughout their careers and are highly sought after for their expertise in their fields. Many within the Integrum family continue to conduct pivotal research and development on emerging and re-emerging infectious diseases by focusing on prevention, preparedness, and awareness. www.integrumsci.com
Media Contacts
CEPI
Email: [email protected]
Phone: +44 7387 055214
Integrum Scientific
Email: [email protected]
Phone: +1 800 660 0924