CEPI and SK bioscience partner to advance mRNA vaccine technology to build vaccine library, enable rapid response against Disease X

October 25, 2022; OSLO: CEPI, the Coalition for Epidemic Preparedness Innovations, and SK bioscience today announced a new partnership agreement to advance the development of mRNA-based vaccine technologies to facilitate rapid response to unknown pathogens with epidemic and pandemic potential (also referred to as Disease X).
CEPI will provide up to US$40 million in initial funding to support the development of mRNA-vaccine candidates against Lassa Fever virus (viral family: Arenaviridae) and Japanese Encephalitis virus (Flaviviridae). The funding will support preclinical studies through to phase 1/2 trials. Pending results of these studies, a further $100 million in funding could be made available to support late-stage trials/licensure to further validate the mRNA platform and have it ready for use in outbreak situations.
This partnership is the first such agreement to be announced under CEPI's call for proposals, launched in January, 2022, to develop RNA vaccine platform technologies and support development of a vaccine library against emerging and specific endemic infectious diseases.
Creating a "vaccine library" ready to use against Disease X
It is possible to develop prototype vaccines against different virus families that infect humans. These prototype vaccines can be based on rapid response platforms, such as mRNA, which can be adapted in a matter of weeks once a new virus has been identified.
CEPI aims to form a ‘library' of vaccine candidates that are ready to be pulled off the shelf and swiftly adapted next time Disease X emerges. That way, valuable time is not lost creating a new vaccine from scratch when a new viral threat emerges.
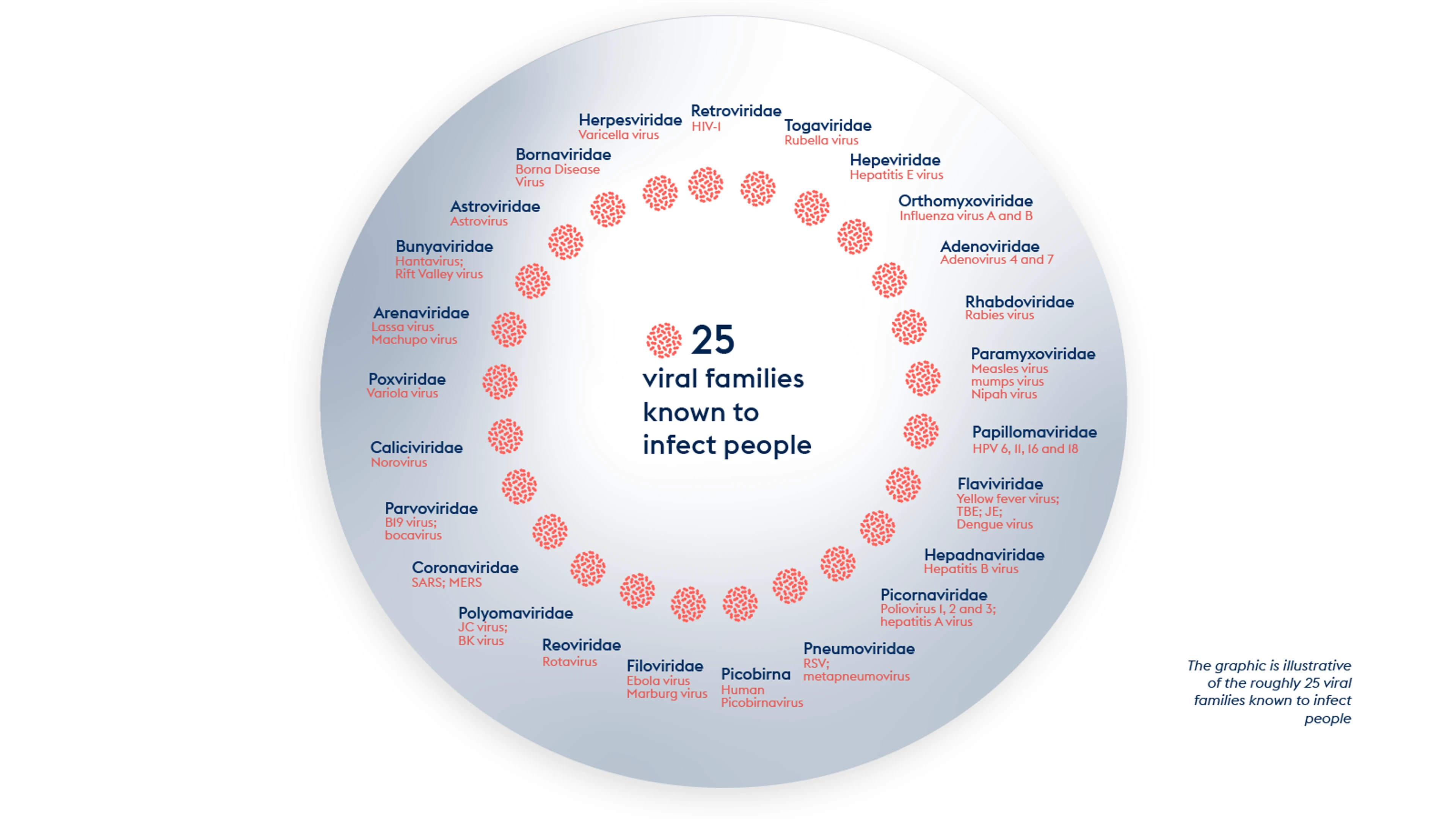
Viral families known to cause disease in people
By supporting the development of mRNA vaccine platform technologies against prototype pathogens, CEPI aims to expand the use of mRNA vaccine platforms and apply this technology to help create a library of vaccine candidates that could be used against Disease X that might emerge from high priority virus families.
CEPI's efforts to help the world develop such a vaccine library, including rapid response technologies, form part of its $3.5 billion plan to compress vaccine development timelines to 100 days: an aspiration that CEPI refers to as the 100 Days Mission, which has been backed by leaders of the G7 and G20.
When the next pandemic virus emerges, we will be racing against the clock. And we are racing against the clock now, because we don't know when the next pandemic virus will emerge. The fact that we have just had a pandemic does not reduce our future risk at all. South Korea's world-leading life-sciences industry can and must continue to play a pivotal role in strengthening global health security and help to create a future free of pandemics. Key to making this future a reality is the ability to rapidly respond to the next Disease X with new vaccines and other countermeasures–in just 100 days. CEPI's expanded partnership with SK bioscience will help kick start the world's efforts to validate these mRNA platform technologies so that they can be used to create a library of vaccines ready for use against the next Disease X, bringing us another step closer achieving the 100 Days Mission, and preparing the world for the next pandemic.
CEPI's partnerships with SK bioscience
To date, CEPI has invested up to $300 million—including the funding announced today—in partnerships with SK bioscience to develop a COVID-19 vaccine, a COVID-19 variant-proof vaccine, a broadly protective Betacoronavirus vaccine, and now mRNA-based vaccine technologies.
We all agree that speed is the most important factor to protect humanity from the next life-threatening pandemic. Based on cooperation with global initiatives, including CEPI, we will achieve innovative vaccine development and ultimately contribute to promoting global public health.
Enabling equitable access to vaccines
CEPI and SK bioscience are committed to enabling global equitable access to the vaccines they develop. Under the terms of the funding agreement, SK bioscience has committed to achieving equitable access to the outputs of this project including prioritisation of supply for low-income and middle-income countries, production of vaccine volumes required to meet public health needs, and affordable pricing, in line with CEPI's Equitable Access Policy.
—END—
About CEPI
CEPI is an innovative partnership between public, private, philanthropic, and civil organisations, launched at Davos in 2017, to develop vaccines against future epidemics. Prior to COVID-19, CEPI's work focused on developing vaccines against Ebola virus, Lassa virus, Middle East Respiratory Syndrome coronavirus, Nipah virus, Rift Valley Fever virus and Chikungunya virus — it has over 20 vaccine candidates against these pathogens in development. CEPI has also invested in new platform technologies for rapid vaccine development against unknown pathogens (Disease X).
During the current pandemic, CEPI initiated multiple programmes to develop vaccines against SARS-CoV-2 and its variants with a focus on speed, scale and access. These programmes leverage the rapid response platforms developed by CEPI's partners prior to the emergence of COVID-19 as well as new collaborations. The aim is to advance clinical development of a diverse portfolio of safe and effective COVID-19 candidates and to enable fair allocation to these vaccines worldwide through COVAX.
CEPI's 5-year plan lays out a $3.5 billion roadmap to compress vaccine development timelines to 100 days, develop a universal vaccine against COVID-19 and other Betacoronaviruses, and create a "library" of vaccine candidates for use against known and unknown pathogens. The plan is available at endpandemics.cepi.net.
Follow our news page for the latest updates. Follow us @CEPIvaccines, @DrRHatchett, and LinkedIn.
About SK bioscience
SK bioscience is a global innovative vaccine and biotech company, standing committed to global pandemic preparedness in vaccine development and manufacturing to create more equitable access to vaccines. In leveraging strengths on cutting-edge vaccine development technologies, SK bioscience has been dedicated to promoting human health from prevention to cure across the globe. Under collaborations of domestic and international governments, regulatory agencies, healthcare providers, doctors and medical experts, SK bioscience has firmly established globally certified R&D and manufacturing technologies. All of the SK colleagues are passionately committed to providing high-quality vaccines to those who need them and better public healthcare solutions.
For further information, please visit: https://www.skbioscience.co.kr/en/main
Media Contacts
CEPI
Email: [email protected]
Phone: +44 7387 055214
SK bioscience
Changhyun Jin ([email protected]) + 82 10-2041-8010
Jeannie Pak ([email protected]) + 82 10-2079-7981
Tae-Gyun Kim ([email protected]) +82 10-9493-0630