Discover the latest CEPI news
Keep up-to-date on the latest announcements and updates from CEPI and our global partners.


News
CEPI backs updated Zaire ebolavirus vaccine that aims to improve vaccine affordability and accessibility
$30 million to update manufacturing process and make a vaccine intended to be less costly to produce and easier to deploy.

News
CEPI, Oxford, Serum create largest-ever reserve of investigational Rift Valley fever vaccine

News
Ambitious research to develop multivalent vaccines against deadly filoviruses
News
CEPI calls for experts to join its Scientific Advisory Committee
.webp)
Blog
Reflections on 2025 – Richard Hatchett
.webp)
News
CEPI to Fund Pivotal Phase 3 Trial for Moderna’s mRNA Pandemic Influenza Candidate

Blog
The pain after the bite: Tracking Chikungunya’s debilitating impact in East Africa

News
Sinergium paves the way for Latin American mRNA vaccines with new preclinical study
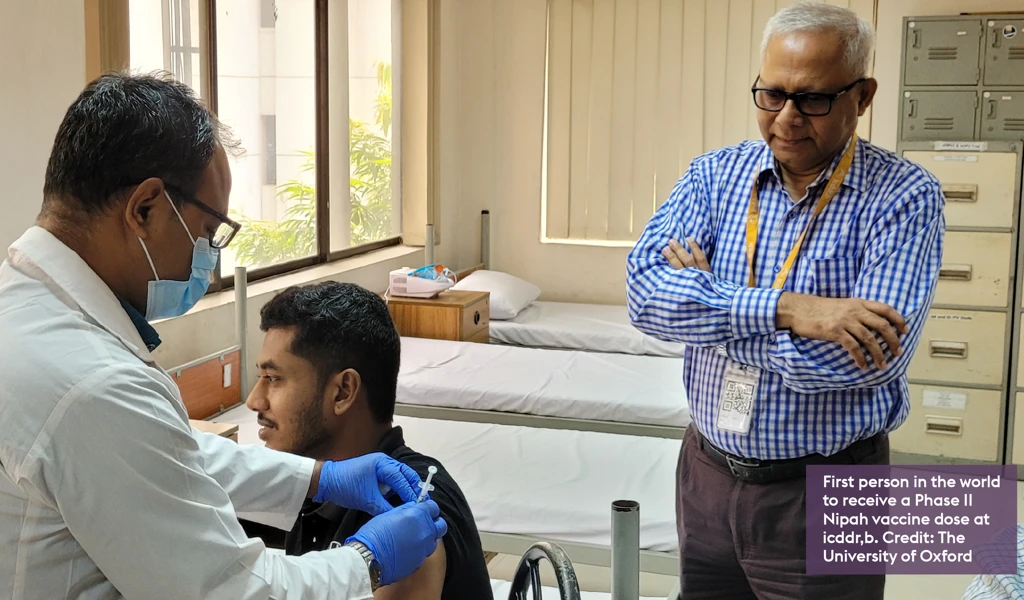
News
University of Oxford launches world’s first Phase II Nipah virus vaccine trial

Impact story
Closing in on protection against deadly Nipah virus

News
Scientists in Korea to create mRNA vaccine against emerging Asian tick-borne virus

Blog
Disease outbreak survivors are key to future preparedness
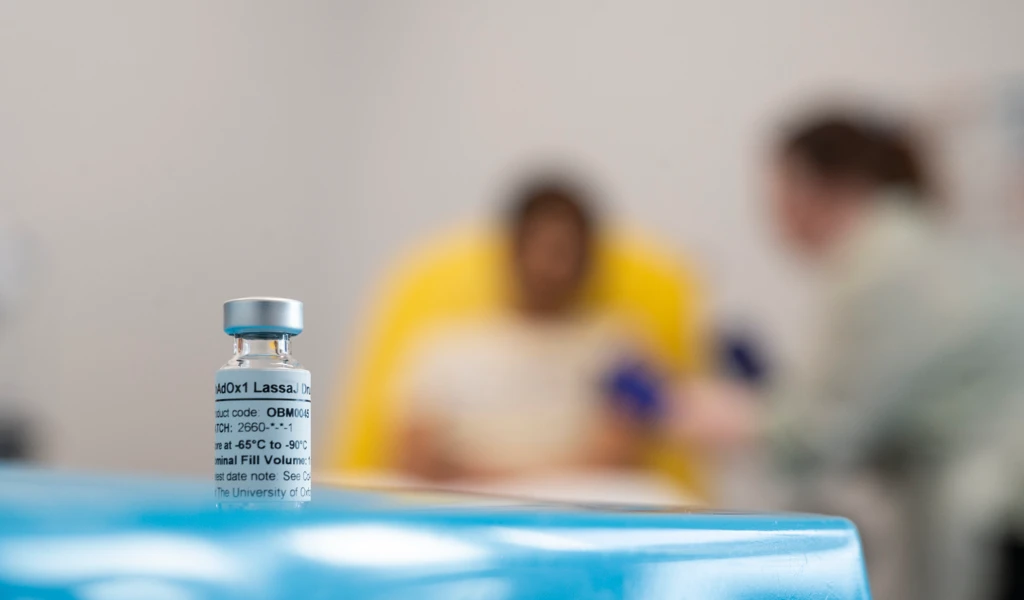
News
First volunteer receives Lassa fever vaccine in cutting-edge Oxford trial
.webp)
News
Korea steps up pandemic response with $18.9m investment in CEPI
Blog
A blueprint for access at the heart of outbreak R&D
News
IAVI’s Lassa fever vaccine shows promise in early clinical trials

News
Progress towards regionalised vaccine manufacturing remains uneven
Blog
The ‘Big One’ is coming: How can we prepare for a future coronavirus pandemic?
.webp)
News
Global vaccine safety network to launch in six countries across three continents

News
Establishing the world’s largest Nipah virus vaccine reserve
 .webp)
News
Serum Institute of India and CEPI supercharge pandemic response preparedness targeting H5N1

News
BMFTR boosts international vaccine initiative CEPI

Blog
Stronger, faster, fairer: making mpox vaccines accessible

News
CEPI partners with AVAREF to boost clinical trial application reviews in Africa
News
CEPI backs new research into vaccines against multiple deadly filoviruses

News
New study shows all-in-one coronavirus vaccines could save millions of lives in future pandemics

Artificial Intelligence
Securing The Pandemic Preparedness Engine
Artificial Intelligence
Building a global AI platform for pandemic preparedness

News
Top health and science experts join coalition preparing for future pandemic threats
News
New Mpox vaccine study to launch in outbreak-affected DRC

News
West African leaders commit to advance Lassa Fever vaccine for the region
News
New global collaboration uses experts and AI to spot the next pandemic

News
Landmark African-led research to map the extent of Rift Valley fever impact

News
Leading science labs in Korea join world’s largest vaccine testing network

News
World-first library of vaccine-enhancing adjuvants launches

Blog
100 Days Mission: are we ready for the next pandemic?

News
New protein vaccine approach could enable faster responses to Disease X

News
Fighting outbreaks with FEEVA: New project could support faster approvals of vaccines
News
R&D experts to create playbooks that map immune markers in deadly diseases

Impact story
Getting ahead of pandemic threats with better, faster, AI vaccine design

News
