Chikungunya
CEPI is advancing the development of promising Chikungunya vaccine candidates through late-stage trials, focusing on expanding access to vulnerable populations in endemic countries.
What is Chikungunya virus?
Chikungunya is a viral disease caused by the Chikungunya virus. It belongs to the Togavirus family.
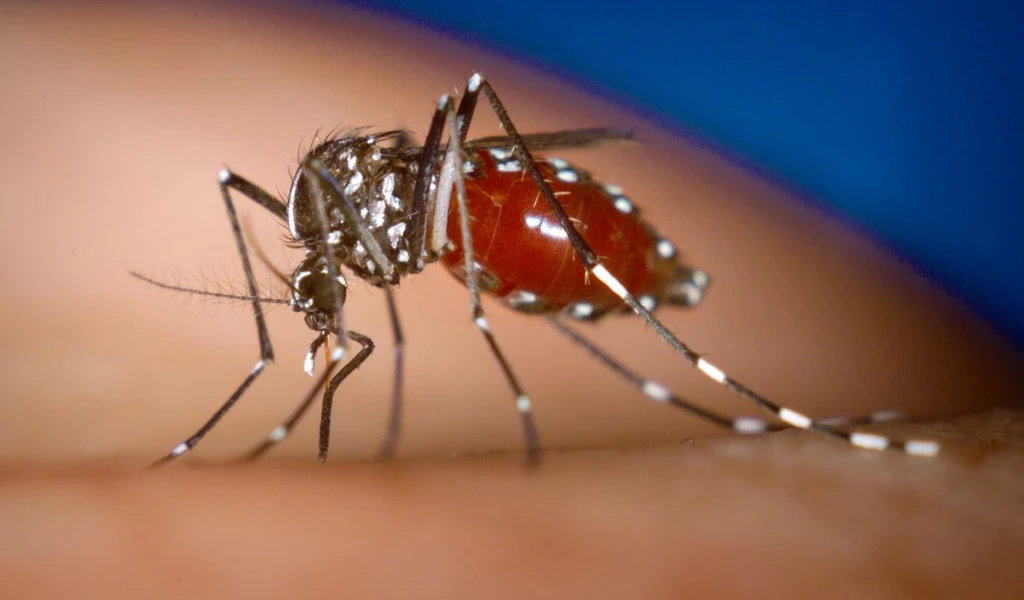
Chikungunya is most commonly transmitted by female Aedes aegypti and Aedes albopictus mosquitoes.
The name chikungunya derives from a word in the Kimakonde language, meaning “to become contorted”.
WHO has identified Chikungunya as a major public health concern due to its high morbidity and has stated that further research and development is needed to mitigate the risk it poses.
In November 2023, the FDA licensed the first-ever Chikungunya vaccine, developed by Valneva with support from CEPI, with support from the European Union’s Horizon programme.

1 billion
Number of people living in areas where Chikungunya is endemic
110+
Number of countries in which the Chikungunya virus has been identified
2
CEPI-backed vaccines in active development, including one that is now licensed
Where does Chikungunya occur?
Chikungunya was first identified in Tanzania in 1952 and has since become widely distributed across the globe.
Since 2004, major epidemics have been reported in Africa and Asia, and local transmission is also now being reported in Europe, the USA and the Caribbean.
Today, over a billion people live in areas where Chikungunya is endemic.
Climate change could further amplify the threat posed by Chikungunya by making more regions habitable for the mosquito vectors that transmit the virus, thereby increasing the size of the population at risk of infection.
What are the symptoms of Chikungunya infection?
Chikungunya symptoms can range widely in terms of severity.
It is typically characterised by fever and severe joint pain, which is often debilitating and can last for weeks, months or even years, severely impacting quality of life. Other symptoms can include muscle pain, headache, nausea, fatigue, and rash.
Most patients recover fully. However, cases of eye, heart, and neurological complications have been reported.
Newborns and older people with underlying medical conditions are at higher risk of severe disease and death.
How is CEPI responding to Chikungunya?
CEPI’s aim is to advance Chikungunya candidate vaccines towards licensure, and enable equitable access for populations living in affected countries.
To date, we have supported three Chikungunya vaccine candidates in late-stage development, committing up to US$58.5 million in funding with support from the European Union’s Horizon programme.
Two of these candidates remain in active development, one of which has become the first in the world to be approved by a Stringent Regulatory Authority and is now pursuing licensure in Brazil with CEPI’s support.
To help address the outstanding R&D questions and, ultimately, support the roll out of Chikungunya vaccines in endemic countries, CEPI and the European Commission have issued a Call for Proposals, worth up to EUR 50 million, to fund effectiveness studies and further scientific studies to help expand the potential use of licensed Chikungunya vaccines for children, people who are immunocompromised, and pregnant women.

Latest Chikungunya news
CEPI awards up to US$21 million to Themis Bioscience for Phase 3 Chikungunya Vaccine Development
The world needs a Chikungunya vaccine
CEPI awards up to US$23.4 million to Valneva for late-stage development of a single-dose Chikungunya vaccine

CEPI awards up to US $14.1 million to consortium of IVI and Bharat Biotech to advance development of Chikungunya vaccine in collaboration with Ind-CEPI
CEPI partners, IVI and BBIL, launch global Chikungunya vaccine Phase II/III trial in Costa Rica

CEPI launches call for proposals to develop vaccines against Rift Valley fever and Chikungunya viruses

